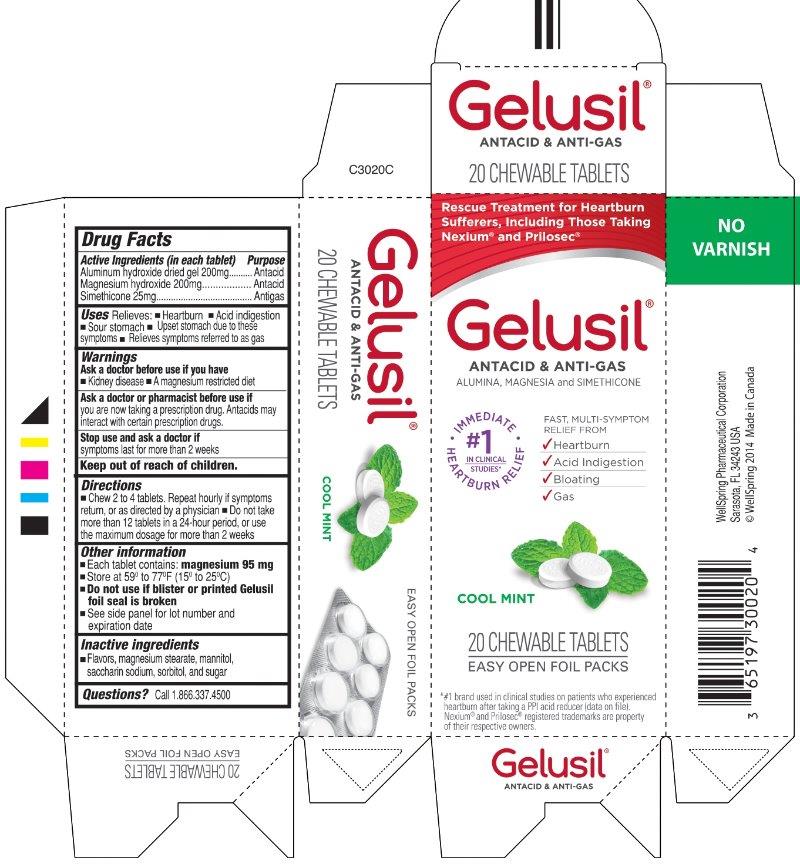
GELUSIL- aluminum hydroxide magnesium hydroxide simethicone tablet, chewable
WellSpring Pharmaceutical Corporation
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Gelusil Tablets
Active Ingredients (in each tablet)
Aluminum hydroxide dried gel 200 mg
Magnesium hydroxide 200 mg
Simethicone 25 mg
Purpose
Active ingredients (in each tablet) Purpose
Aluminum hydroxide dried gel 200 mg........................Antacid
Magnesium hydroxide 200 mg.....................................Antacid
Simethicone 25 mg......................................................Antigas
Uses
Relieves:
- heartburn
- acid indigestion
- sour stomach
- upset stomach due to these symptoms
- relieves symptoms referred to as gas
Warnings
Directions
- Chew 2 to 4 tablets. Repeat hourly if symptoms return, or as directed by a physician.
- Do not take more than 12 tablets in a 24-hour period, or use the maximum dosage for more than 2 weeks
| GELUSIL
aluminum hydroxide magnesium hydroxide simethicone tablet, chewable |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - WellSpring Pharmaceutical Corporation (110999054) |
Revised: 8/2014
Document Id: 87e19f8e-57ae-40b9-ba8f-6003339748e5
Set id: bddf72bf-985e-45c7-b37a-026687fa5fd1
Version: 8
Effective Time: 20140804
WellSpring Pharmaceutical Corporation