Label: CHLORHEXIDINE GLUCONATE cloth
- NDC Code(s): 53462-705-20, 53462-705-23, 53462-705-26
- Packager: Sage Products LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated May 24, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
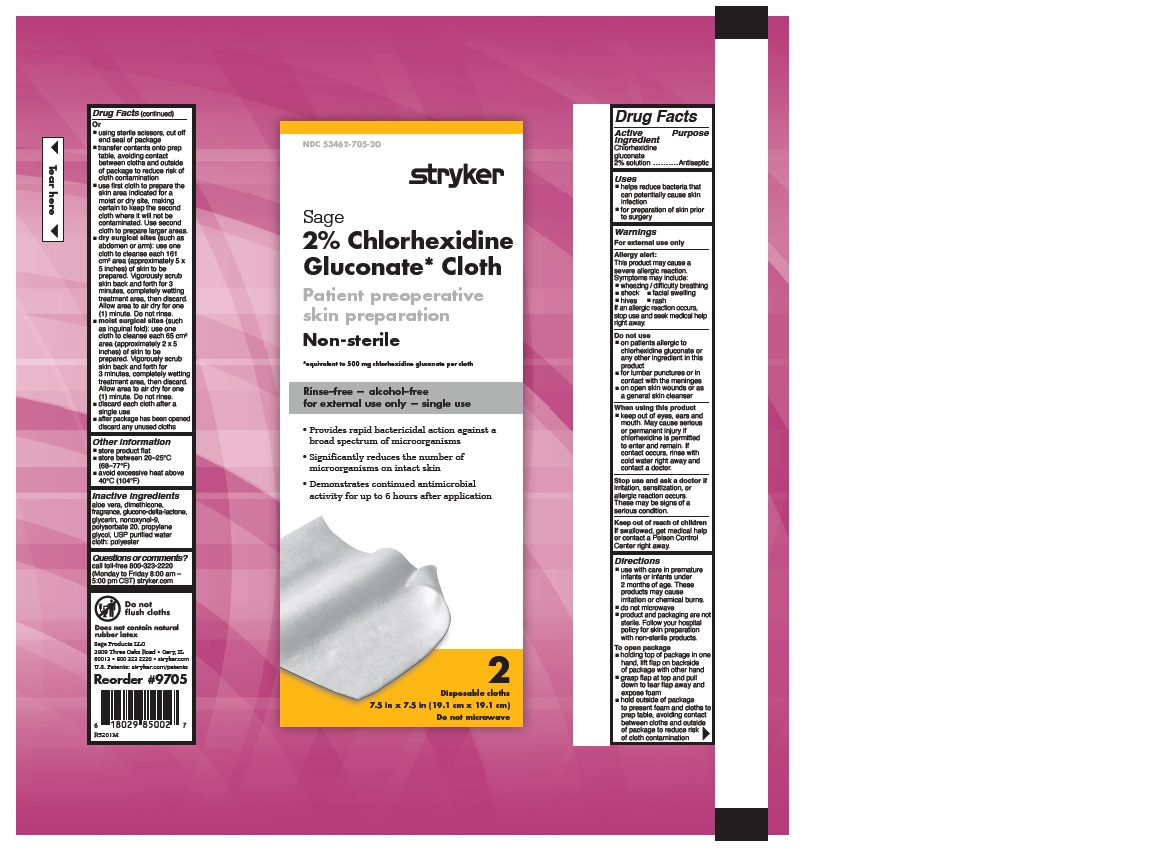
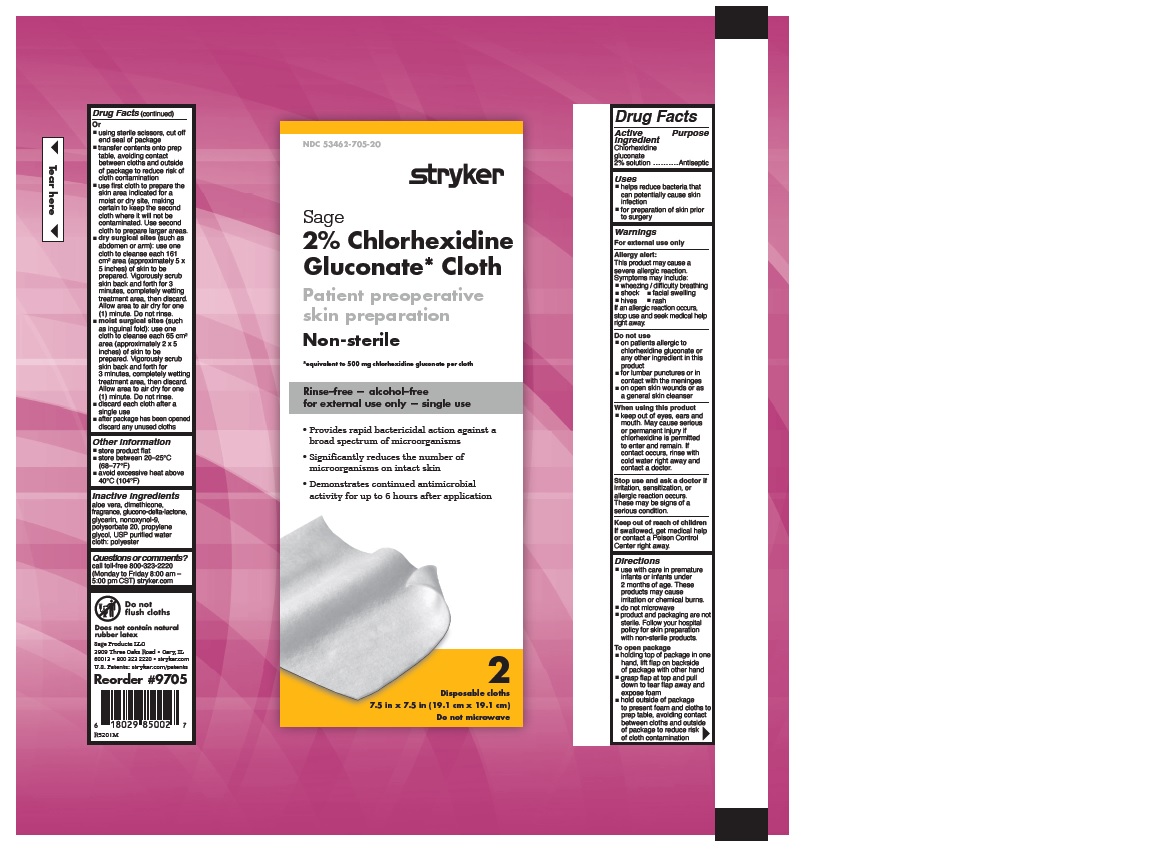
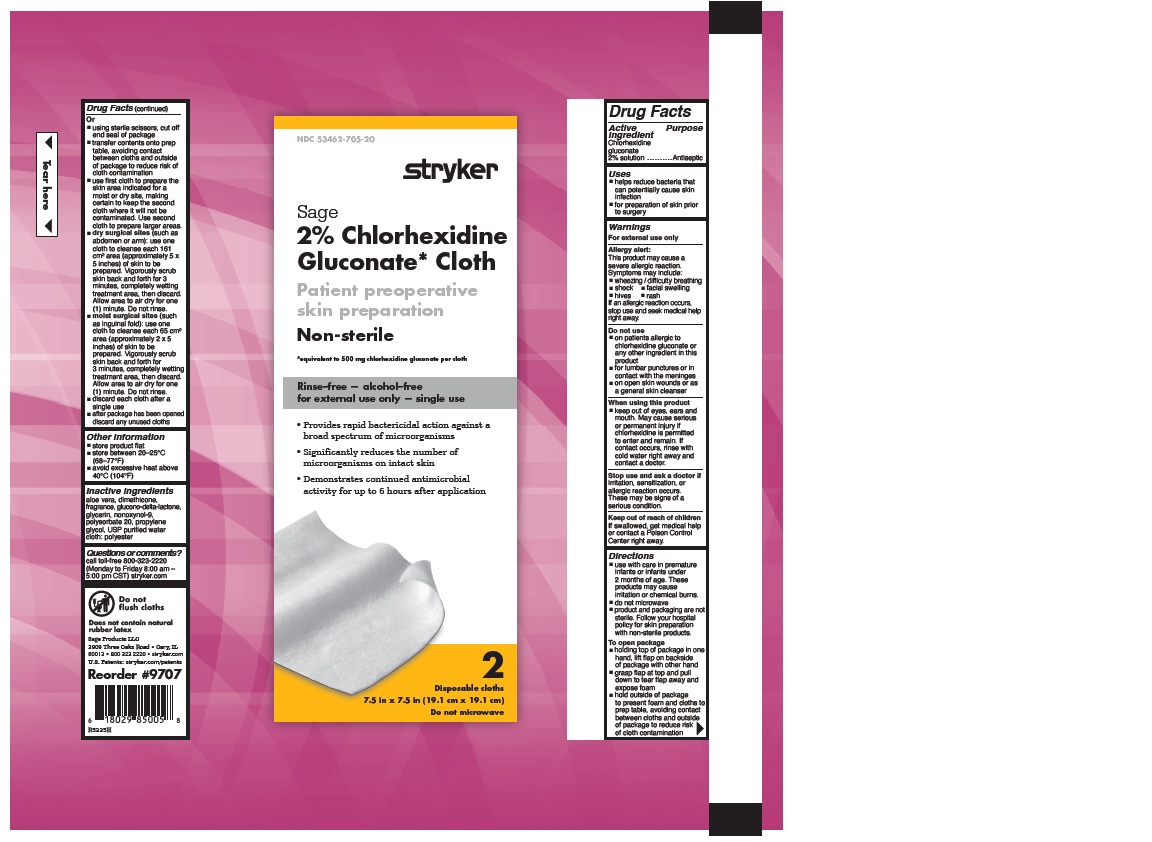
- 2% CHLORHEXIDINE GLUCONATE CLOTH
- PURPOSE
- Uses
-
Warnings
Allergy alert:
This product may cause a severe allergic reaction. Symptoms may include:
- wheezing/difficulty breathing
- shock
- facial swelling
- hives
- rash
If an allergic reaction occurs, stop use and seek medical help right away.
- on patients allergic to chlorhexidine gluconate or any other ingredient in this product
- for lumbar punctures or in contact with the meninges
- on open skin wounds or as a general skin cleanser
- keep out of eyes, ears and mouth. May cause serious or permanent injury if chlorhexidine is permitted to enter and remain. If contact occurs, rinse with cold water right away and contact a doctor.
Stop use and ask a doctor if irritation, sensitization or allergic reaction occurs. These may be signs of a serious condition.
- Keep out of reach of children
-
Directions
- use with care in premature infants or infants under 2 months of age. These products may cause irritation or chemical burns.
- do not microwave
- product and packaging are not sterile. Follow your hospital policy for skin preparation with non-sterile products.
- holding top of package in one hand, lift flap on backside of package with other hand
- grasp flap at top and pull down to tear flap away and expose foam
- hold outside of package to present foam and cloths to prep table, avoiding contact between cloths and outside of package to reduce risk of cloth contamination
- using sterile scissors, cut off end seal of package
- transfer contents onto prep table, avoiding contact between cloths and outside of package to reduce risk of cloth contamination
- use first cloth to prepare the skin area indicated for a moist or dry site, making certain to keep the second cloth where it will not be contaminated. Use second cloth to prepare larger areas.
- dry surgical sites (such as abdomen or arm): use one cloth to cleanse each 161 cm 2 area (approximately 5 x 5 inches) of skin to be prepared. Vigorously scrub skin back and forth for 3 minutes, completely wetting treatment area, then discard. Allow area to air dry for one (1) minute. Do not rinse.
- moist surgical sites (such as inguinal fold): use one cloth to cleanse each 65 cm 2 area (approximately 2 x 5 inches) of skin to be prepared. Vigorously scrub skin back and forth for 3 minutes, completely wetting treatment area, then discard. Allow area to air dry for one (1) minute. Do not rinse.
- discard each cloth after a single use
- after package has been opened discard any unused cloths
- Other information
- Inactive Ingredients
- Questions or comments?
- 2 pack
- 3 x 2 packs
- 6 pack
-
INGREDIENTS AND APPEARANCE
CHLORHEXIDINE GLUCONATE
chlorhexidine gluconate clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:53462-705 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) (CHLORHEXIDINE - UNII:R4KO0DY52L) CHLORHEXIDINE GLUCONATE 500 mg Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) NONOXYNOL-9 (UNII: 48Q180SH9T) POLYSORBATE 20 (UNII: 7T1F30V5YH) DIMETHICONE 350 (UNII: 2Y53S6ATLU) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ALOE VERA LEAF (UNII: ZY81Z83H0X) GLYCERIN (UNII: PDC6A3C0OX) GLUCONOLACTONE (UNII: WQ29KQ9POT) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53462-705-20 2 in 1 PACKAGE; Type 0: Not a Combination Product 02/01/2006 2 NDC:53462-705-23 6 in 1 PACKAGE; Type 0: Not a Combination Product 02/01/2006 3 NDC:53462-705-26 6 in 1 PACKAGE; Type 0: Not a Combination Product 10/31/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021669 02/01/2006 Labeler - Sage Products LLC (054326178) Registrant - Sage Products LLC (054326178)