LORATADINE- loratadine tablet
GlaxoSmithKline Consumer Healthcare Holdings (US) LLC
----------
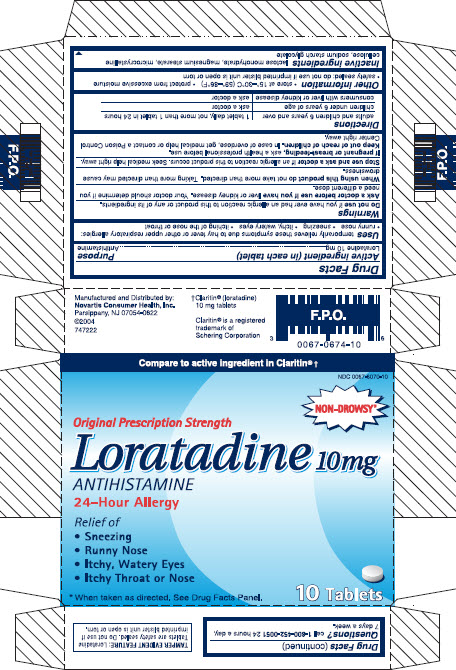
Drug Facts
Uses
Temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
● runny nose
● sneezing
● itchy, watery eyes
● itching of the nose or throat
Warnings
Ask doctor before use if you have liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product do not take more than directed. Taking more than directed may cause drowsiness.
Other information
● store at 15-30C (59-86F)
● protect from excessive moisture
● safety sealed: do not use if imprinted blister unit is open or torn
| LORATADINE
loratadine tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - GlaxoSmithKline Consumer Healthcare Holdings (US) LLC (079944263) |
Revised: 1/2018
Document Id: 816a59c0-7179-4266-935f-03f3323fa690
Set id: bd385a9f-f82f-4965-83fd-273c795f9b4f
Version: 2
Effective Time: 20180116
GlaxoSmithKline Consumer Healthcare Holdings (US) LLC