COUGH AND COLD- chlorpheniramine maleate and dextromethorphan hbr tablet, sugar coated
CVS Pharmacy
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
CVS 44-411-Delisted
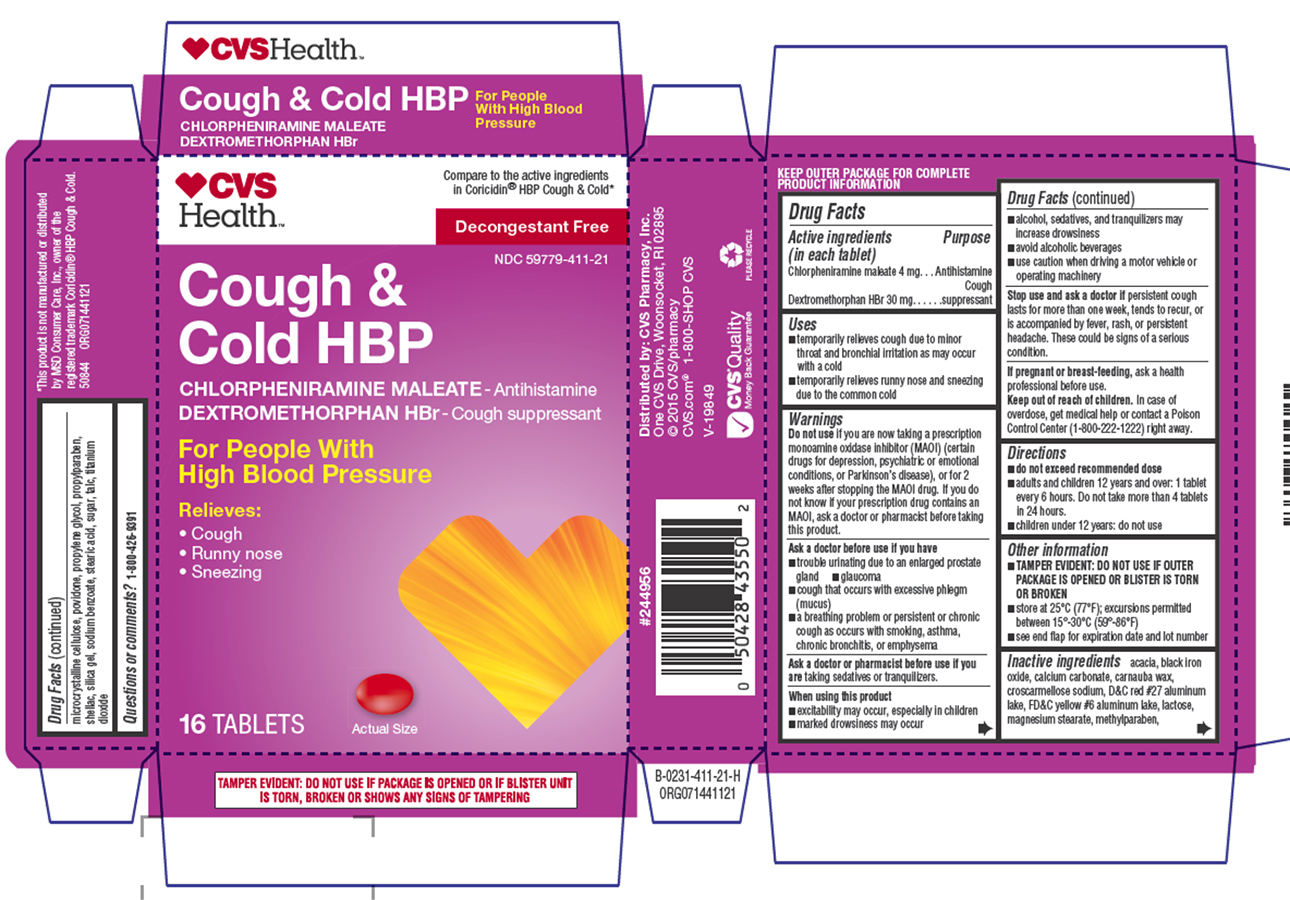
Uses
- temporarily relieves cough due to minor throat and bronchial irritation as may occur with a cold
- temporarily relieves runny nose and sneezing due to the common cold
Warnings
Do not use
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- trouble urinating due to an enlarged prostate gland
- glaucoma
- cough that occurs with excessive phlegm (mucus)
- a breathing problem or persistent or chronic cough as occurs with smoking, asthma, chronic bronchitis, or emphysema
When using this product
- excitability may occur, especially in children
- marked drowsiness may occur
- alcohol, sedatives, and tranquilizers may increase drowsiness
- avoid alcoholic beverages
- use caution when driving a motor vehicle or operating machinery
Directions
- do not exceed recommended dose
- adults and children 12 years and over: 1 tablet every 6 hours. Do not take more than 4 tablets in 24 hours.
- children under 12 years: do not use
Other information
- TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN
- store at 25ºC (77ºF); excursions permitted between 15º-30ºC (59º-86ºF)
- see end flap for expiration date and lot number
Inactive ingredients
acacia, black iron oxide, calcium carbonate, carnauba wax, croscarmellose sodium, D&C red #27 aluminum lake, FD&C yellow #6 aluminum lake, lactose, magnesium stearate, methylparaben, microcrystalline cellulose, povidone, propylene glycol, propylparaben, shellac, silica gel, sodium benzoate, stearic acid, sugar, talc, titanium dioxide
Principal Display Panel
CVS
Health™
Compare to the active ingredients
in Coricidin® HBP Cough & Cold*
Decongestant Free
NDC 59779-411-21
Cough &
Cold HBP
CHLORPHENIRAMINE MALEATE - Antihistamine
DEXTROMETHORPHAN HBr - Cough suppressant
For People With
High Blood Pressure
Relieves:
• Cough
• Runny Nose
• Sneezing
16 TABLETS
Actual Size
TAMPER EVIDENT: DO NOT USE IF PACKAGE IS OPENED OR IF BLISTER UNIT
IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING
*This product is not manufactured or distributed
by MSD Consumer Care, Inc., owner of the
registered trademark Coricidin® HBP Cough & Cold.
50844 ORG071441121
Distributed by: CVS Pharmacy, Inc.
One CVS Drive, Woonsocket, RI 02895
© 2015 CVS/pharmacy
CVS.com® 1-800-SHOP CVS
V-19849
✓ CVS® Quality
Money Back Guarantee
CVS 44-411
| COUGH AND COLD
chlorpheniramine maleate and dextromethorphan hbr tablet, sugar coated |
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - CVS Pharmacy (062312574) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867894 | MANUFACTURE(59779-411) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867837 | PACK(59779-411) | |