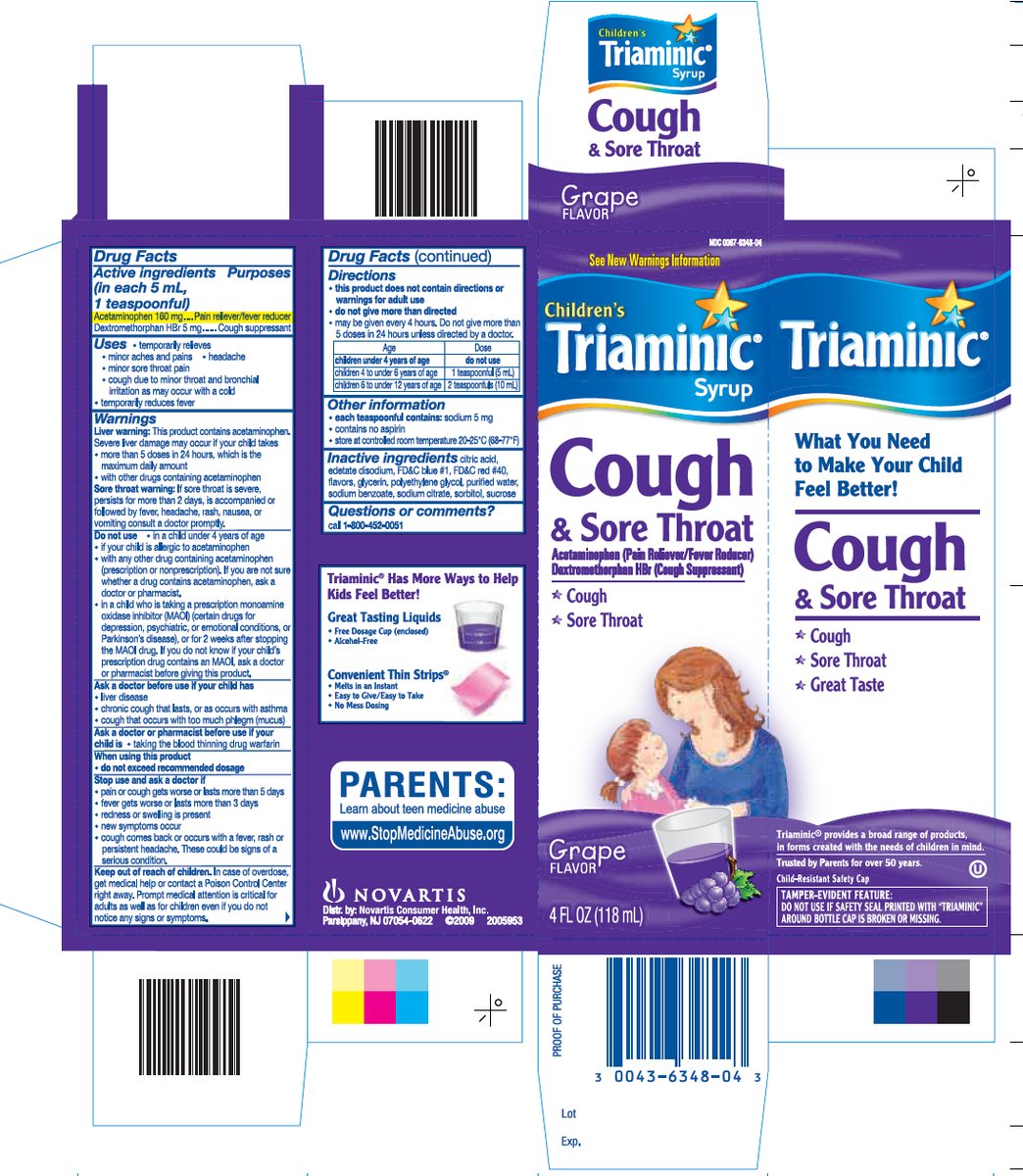
TRIAMINIC COUGH AND SORE THROAT- acetaminophen, dextromethorphan hbr syrup
GlaxoSmithKline Consumer Healthcare Holdings (US) LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Uses
- •
- temporarily relieves minor aches and pains
- •
- minor aches and pains
- •
- headache
- •
- minor sore throat pain
- •
- cough due to minor throat and bronchial irritation as may occur with a cold
- •
- temporarily reduces fever
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if your child takes
- •
- more than 5 doses in 24 hours, which is the maximum daily amount
- •
- with other drugs containing acetaminophen
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- •
- in a child under 4 years of age
- •
- if your child is allergic to acetaminophen
- •
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- •
- in a child who is taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your child’s prescription drug contains an MAOI, ask a doctor or pharmacist before giving this product.
Ask a doctor before use if your child has
- •
- liver disease
- •
- chronic cough that lasts, or as occurs with asthma
- •
- cough that occurs with too much phlegm (mucus)
Directions
- •
- this product does not contain directions or complete warnings for adult use
- •
- do not give more than directed
- •
- may be given every 4 hours. Do not give more than 5 doses in 24 hours unless directed by a doctor.
- •
- find the right dose on chart below.
- •
- use enclosed dosing cup only. Keep for use with this product only. Do not use any other dosing device.
|
Age |
Dose |
|
Children under 4 years of age |
do not use |
|
Children under 4 to under 6 years of age |
1 teaspoonful (5 mL) |
|
Children under 6 to under 12 years of age |
2 teaspoonful (10 mL) |
Other information
- •
- each teaspoonful contains: sodium 5 mg
- •
- contains no aspirin
- •
- store at controlled room temperature 20-25°C (68-77°F)
Inactive ingredients
citric acid, edetate disodium, FD&C blue #1, FD&C red #40, flavors, glycerin, polyethylene glycol, purified water, sodium benzoate, sodium citrate, sorbitol, sucrose
Additional information listed on other panels
PARENTS: Learn about teen medicine abuse.
www.StopMedicineAbuse.org
Child-Resistant Safety Cap
TAMPER-EVIDENT FEATURE:
DO NOT USE IF SAFETY SEAL IMPRINTED WITH “TRIAMINIC”
AROUND BOTTLE CAP IS BROKEN OR MISSING.
Triaminic® Has More Ways to Help Kids Feel Better.
Great Tasting Liquids
- •
- Free Dosage Cup (enclosed)
- •
- Alcohol - Free
Thin Strips®
- •
- Melts in an Instant
- •
- Easy to Give/Easy to Take
- •
- No Mess Dosing
What You Need to Make Your Child Feel Better!
For more information plus helpful tips visit www.triaminic.com
Distr. by: Novartis Consumer Health, Inc.
Parsippany, NJ 07054-0622 ©20XX
| TRIAMINIC
COUGH AND SORE THROAT
acetaminophen, dextromethorphan hbr syrup |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - GlaxoSmithKline Consumer Healthcare Holdings (US) LLC (079944263) |