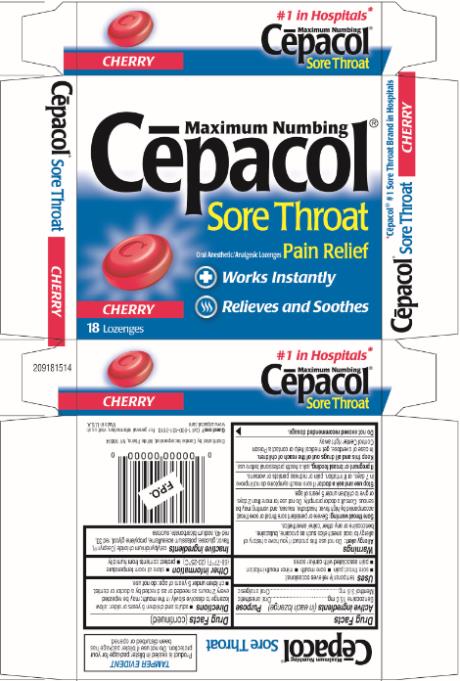
CEPACOL SORE THROAT MAXIMUM NUMBING CHERRY- benzocaine and menthol lozenge
CEPACOL SORE THROAT MAXIMUM NUMBING SUGAR FREE CHERRY- benzocaine and menthol lozenge
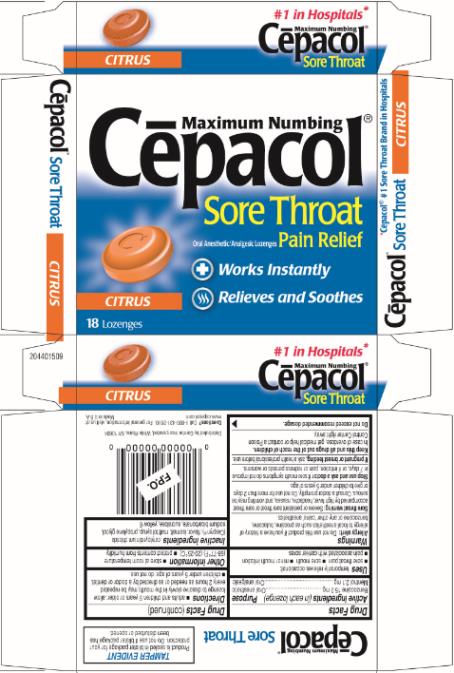
CEPACOL SORE THROAT MAXIMUM NUMBING CITRUS- benzocaine and menthol lozenge
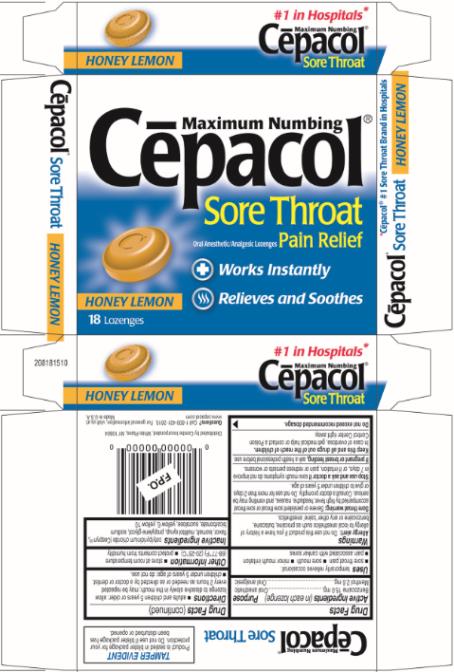
CEPACOL SORE THROAT MAXIMUM NUMBING HONEY LEMON- benzocaine and menthol lozenge
Combe Incorporated
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Cepacol Sore Throat Maximum Numbing
Uses
temporarily relieves occasional:
- sore throat pain
- sore mouth
- minor mouth irritation
- pain associated with canker sores
Warnings
Allergy alert: Do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or any other ‘caine’ anesthetics.
Sore Throat Warning: Severe or persistent sore throat or sore throat accompanied by high fever, headache, nausea, and vomiting may be serious. Consult a doctor promptly. Do not use for more than 2 days or give to children under 5 years of age.
Directions
- adults and children 5 years or older: allow lozenge to dissolve slowly in the mouth; may be repeated every 2 hours as needed or as directed by a doctor or dentist.
- children under 5 years of age: do not use.
Inactive ingredients
cetylpyridinium chloride (Ceepryn®), flavor, glucose, potassium acesulfame, propylene glycol, red 33, red 40, sodium bicarbonate, sucrose
Cēpacol® Sore Throat Maximum Numbing Sugar Free Cherry Lozenges
Uses
temporarily relieves occasional:
- sore throat pain
- sore mouth
- minor mouth irritation
- pain associated with canker sores
Warnings
Allergy alert: Do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or any other ‘caine’ anesthetics.
Sore Throat Warning: Severe or persistent sore throat or sore throat accompanied by high fever, headache, nausea, and vomiting may be serious. Consult a doctor promptly. Do not use for more than 2 days or give to children under 5 years of age.
Directions
- adults and children 5 years or older: allow lozenge to dissolve slowly in the mouth; may be repeated every 2 hours as needed or as directed by a doctor or dentist.
- children under 5 years of age: do not use.
Inactive ingredients
cetylpyridinium chloride (Ceepryn®), flavor, isomalt, maltitol, potassium acesulfame, propylene glycol, red 33, red 40, sodium bicarbonate
Cēpacol® Sore Throat Maximum Numbing Citrus Lozenges
Uses
temporarily relieves occasional:
- sore throat pain
- sore mouth
- minor mouth irritation
- pain associated with canker sores
Warnings
Allergy alert: Do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or any other ‘caine’ anesthetics.
Sore Throat Warning: Severe or persistent sore throat or sore throat accompanied by high fever, headache, nausea, and vomiting may be serious. Consult a doctor promptly. Do not use for more than 2 days or give to children under 5 years of age.
Directions
- adults and children 5 years or older: allow lozenge to dissolve slowly in the mouth; may be repeated every 2 hours as needed or as directed by a doctor or dentist.
- children under 5 years of age: do not use.
Inactive ingredients
cetylpyridinium chloride (Ceepryn®), flavor, isomalt, maltitol syrup, propylene glycol, sodium bicarbonate, sucralose, yellow 6
Cēpacol® Sore Throat Maximum Numbing Honey Lemon Lozenges
Uses
temporarily relieves occasional:
- sore throat pain
- sore mouth
- minor mouth irritation
- pain associated with canker sores
Warnings
Allergy alert: Do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or any other ‘caine’ anesthetics.
Sore Throat Warning: Severe or persistent sore throat or sore throat accompanied by high fever, headache, nausea, and vomiting may be serious. Consult a doctor promptly. Do not use for more than 2 days or give to children under 5 years of age.
Directions
- adults and children 5 years or older: allow lozenge to dissolve slowly in the mouth; may be repeated every 2 hours as needed or as directed by a doctor or dentist.
- children under 5 years of age: do not use.
Inactive ingredients
cetylpyridinium chloride (Ceepryn®), flavor, isomalt, maltitol syrup, propylene glycol, sodium bicarbonate, sucralose, yellow 6, yellow 10.
Principal Display Panel
Maximum Numbing
Cēpacol®
Sore Throat
Oral Anesthetic/Analgesic Lozenges Pain Relief
Works Instantly
Relieves and Soothes
CHERRY
18 Lozenges
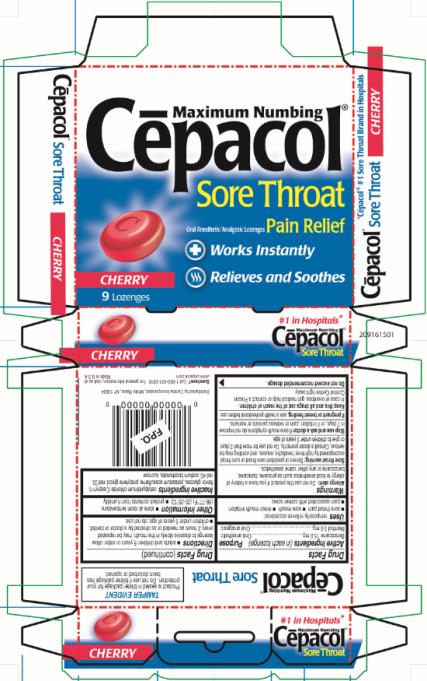
Principal Display Panel
Maximum Numbing
Cēpacol®
Sore Throat
Oral Anesthetic/Analgesic Lozenges Pain Relief
Works Instantly
Relieves and Soothes
CHERRY
9 Lozenges
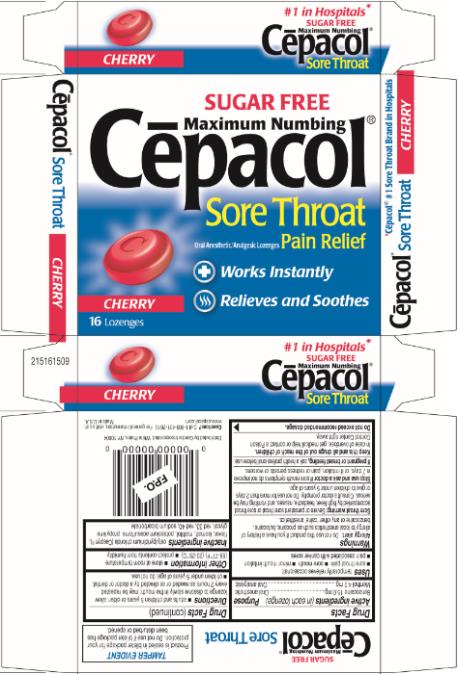
Principal Display Panel
SUGAR FREE
Maximum Numbing
Cēpacol®
Sore Throat
Oral Anesthetic/Analgesic Lozenges Pain Relief
Works Instantly
Relieves and Soothes
CHERRY
16 Lozenges
| CEPACOL SORE THROAT MAXIMUM NUMBING CHERRY
benzocaine and menthol lozenge |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| CEPACOL SORE THROAT MAXIMUM NUMBING SUGAR FREE CHERRY
benzocaine and menthol lozenge |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| CEPACOL SORE THROAT MAXIMUM NUMBING SUGAR FREE CHERRY
benzocaine and menthol lozenge |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| CEPACOL SORE THROAT MAXIMUM NUMBING CITRUS
benzocaine and menthol lozenge |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| CEPACOL SORE THROAT MAXIMUM NUMBING HONEY LEMON
benzocaine and menthol lozenge |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Combe Incorporated (002406502) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| First Boston Pharma LLC | 618613590 | MANUFACTURE(11509-0011, 11509-0014, 11509-0031, 11509-0013, 11509-0012) | |