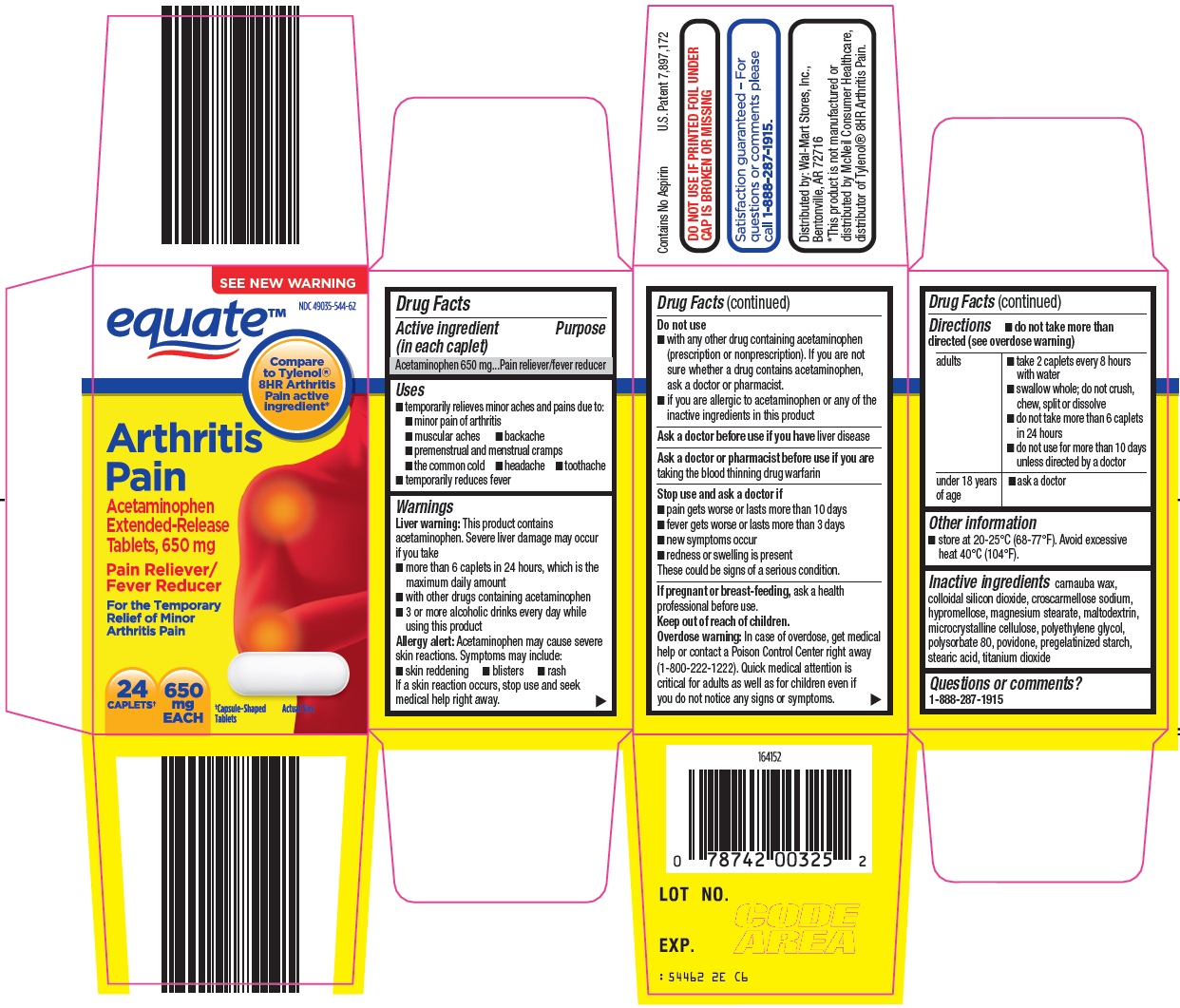
EQUATE ARTHRITIS PAIN- acetaminophen tablet, film coated, extended release
Wal-Mart Stores Inc
----------
Wal-Mart Arthritis Pain Drug Facts
Uses
- •
- temporarily relieves minor aches and pains due to:
- •
- minor pain of arthritis
- •
- muscular aches
- •
- backache
- •
- premenstrual and menstrual cramps
- •
- the common cold
- •
- headache
- •
- toothache
- •
- temporarily reduces fever
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- •
- more than 6 caplets in 24 hours, which is the maximum daily amount
- •
- with other drugs containing acetaminophen
- •
- 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- •
- skin reddening
- •
- blisters
- •
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
- •
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- •
- if you are allergic to acetaminophen or any of the inactive ingredients in this product
Directions
- •
- do not take more than directed (see overdose warning)
|
adults |
|
|
under 18 years of age |
|
| EQUATE ARTHRITIS PAIN
acetaminophen tablet, film coated, extended release |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Wal-Mart Stores Inc (051957769) |
Revised: 6/2019
Document Id: 253dc756-0415-4c72-be59-fc7aecfc0fc7
Set id: baac3c00-a651-4fd8-92f8-d790f1d0b1b7
Version: 7
Effective Time: 20190613
Wal-Mart Stores Inc