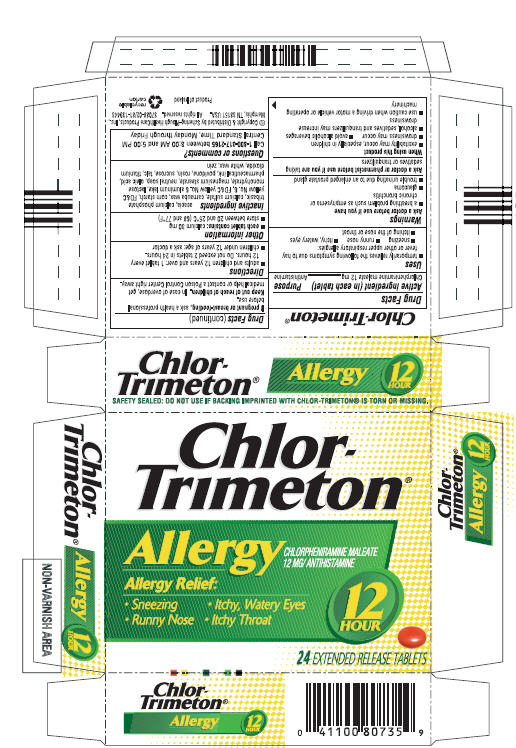
CHLOR-TRIMETON 12 HOUR- chlorpheniramine maleate tablet, extended release
Bayer HealthCare LLC.
----------
Chlor-Trimeton
Uses
- temporarily relieves the following symptoms due to hay fever or other upper respiratory allergies:
- sneezing
- runny nose
- itchy, watery eyes
- itching of the nose or throat
Warnings
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
Directions
- adults and children 12 years and over: 1 tablet every 12 hours. Do not exceed 2 tablets in 24 hours.
- children under 12 years of age: ask a doctor
Inactive ingredients
acacia, calcium phosphate tribasic, calcium sulfate, carnauba wax, corn starch, FD&C yellow No. 6, FD&C yellow No. 6 aluminum lake, lactose monohydrate, magnesium stearate, neutral soap, oleic acid, pharmaceutical ink, povidone, rosin, sucrose, talc, titanium dioxide, white wax, zein
| CHLOR-TRIMETON 12 HOUR
chlorpheniramine maleate tablet, extended release |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - Bayer HealthCare LLC. (112117283) |
Revised: 9/2018
Document Id: 7584ccba-5c52-3ea1-e053-2a91aa0ae088
Set id: ba907153-2639-485b-abd8-af239eccd4ed
Version: 3
Effective Time: 20180910
Bayer HealthCare LLC.