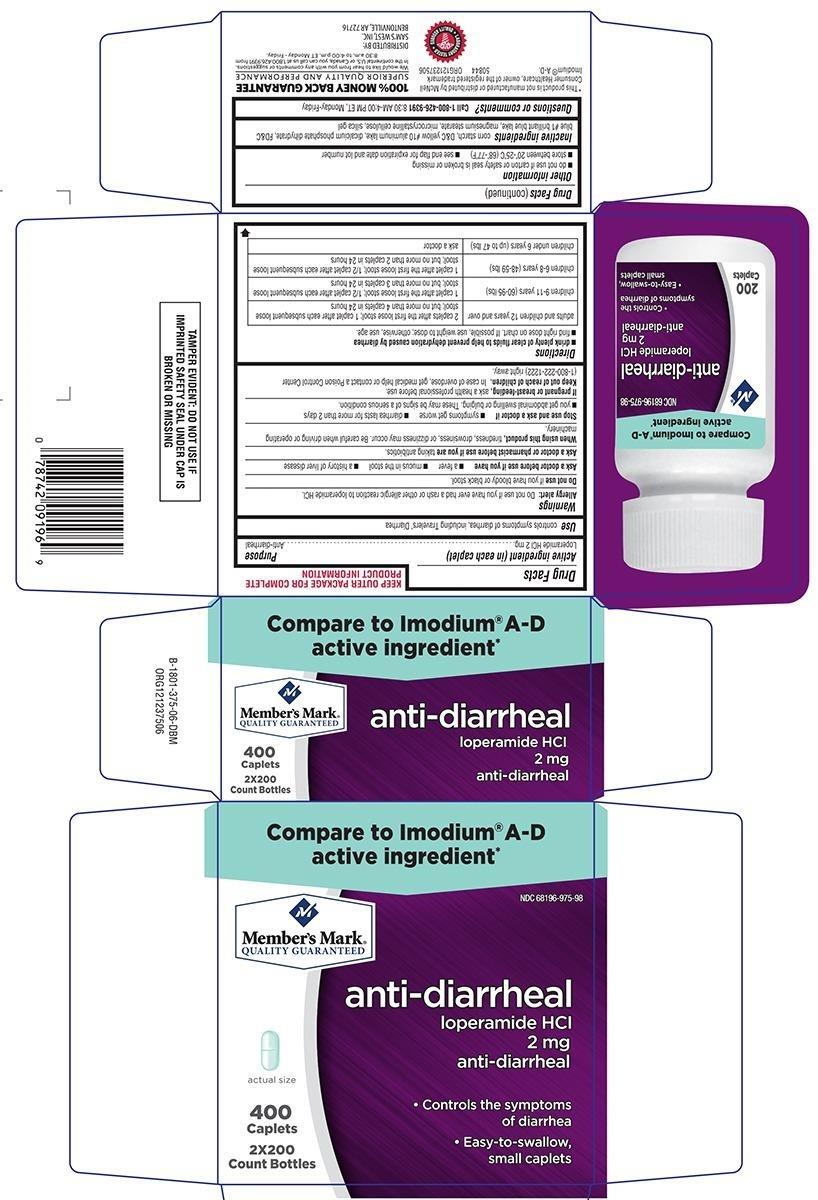
ANTI-DIARRHEAL- loperamide hcl tablet
Sam's West Inc
----------
Members Mark 44-375 Delisted
Warnings
Allergy alert: Do not use if you have ever had a rash or other allergic reaction to loperamide HCl.
When using this product,
tiredness, drowsiness, or dizziness may occur. Be careful when driving or operating machinery.
Directions
- drink plenty of clear fluids to help prevent dehydration caused by diarrhea
- find right dose on chart. If possible, use weight to dose; otherwise, use age.
| adults and children 12 years and over | 2 caplets after the first loose stool;1 caplet after each subsequent loose stool; but no more than 4 caplets in 24 hours |
| children 9-11 years (60-95 lbs) | 1 caplet after the first loose stool; 1/2 caplet after each subsequent loose stool; but no more than 3 caplets in 24 hours |
| children 6-8 years (48-59 lbs) | 1 caplet after the first loose stool; 1/2 caplet after each subsequent loose stool; but no more than 2 caplets in 24 hours |
| children under 6 years (up to 47 lbs) | ask a doctor |
Other information
- do not use if carton or safety seal is broken or missing
- store between 20º-25ºC (68º-77ºF)
- see end flap for expiration date and lot number
Inactive ingredients
corn starch, D&C yellow #10 aluminum lake, dicalcium phosphate dihydrate, FD&C blue #1 brilliant blue lake, magnesium stearate, microcrystalline cellulose, silica gel
Principal Display Panel
Compare to Imodium® A-D
active ingredient*
NDC 68196-975-98
Member's Mark®
QUALITY GUARANTEED
anti-diarrheal
loperamide HCl
2 mg
anti-diarrheal
• Controls the symptoms
of diarrhea
• Easy-to-swallow,
small caplets
actual size
400
Caplets
2x200
Count Bottles
TAMPER EVIDENT: DO NOT USE IF
IMPRINTED SAFETY SEAL UNDER CAP IS
BROKEN OR MISSING
*This product is not manufactured or distributed by McNeil
Consumer Healthcare, owner of the registered trademark
Imodium® A-D. 50844 ORG121237506
100% MONEY BACK GUARANTEE
SUPERIOR QUALITY AND PERFORMANCE
We would like to hear from you with any comments or suggestions.
In the continental U.S. or Canada, you can call us at 1.800.426.9391 from
8:30 a.m. to 4:00 p.m. ET Monday - Friday.
DISTRIBUTED BY:
SAM'S WEST, INC.
BENTONVILLE, AR 72716
Members Mark 44-375
| ANTI-DIARRHEAL
loperamide hcl tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Sam's West Inc (051957769) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 038154464 | PACK(68196-975) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867894 | MANUFACTURE(68196-975) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867837 | PACK(68196-975) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 868734088 | PACK(68196-975) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 967626305 | PACK(68196-975) | |