URINARY PAIN RELIEF- phenazopyridine hydrochloride tablet
Strategic Sourcing Services LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
DRUG FACTS
Uses
for temporary relief of
- urinary pain
- burning
- urgency
- frequency associated with urinary tract infection
Warnings
Ask a doctor before use if you have
- kidney disease
- hepatic or renal problems
- allergies to foods, preservatives, or dyes
- previously exhibited hypersensitivity to Phenazopyridine.
Stop use and ask a doctor if
- symptoms persists for more than 2 days
- yellowish tinge of skin or sclera occurs
Directions
- adults and children 12 years of age and over: take 2 tablets 3 times per day with or after meals as needed. Take with a full glass of water.
- children under 12 years of age: consult a doctor
Other information
- store at controlled room temperature 15°-30°C (59°-86°F)
- do not use if cellophane packet is torn or open
- *This product is not manufactured or distributed by Amerifit Brands, Inc., the distributor of AZO Standard®.
Inactive ingredients
lactose, magnesium silicate, magnesium stearate, microcrystalline cellulose, pharmaceutical glaze and sodium starch glycolate
Principal Display Panel
Compare to AZO STANDARD® active ingredient*
Urinary Pain Relief
Phenazopyridine HCl
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION
Another quality product Distributed by McKesson
One post street, San Franciso, CA 94104
Money back guarantee
Please visit us at www.sunmarkbrand.com
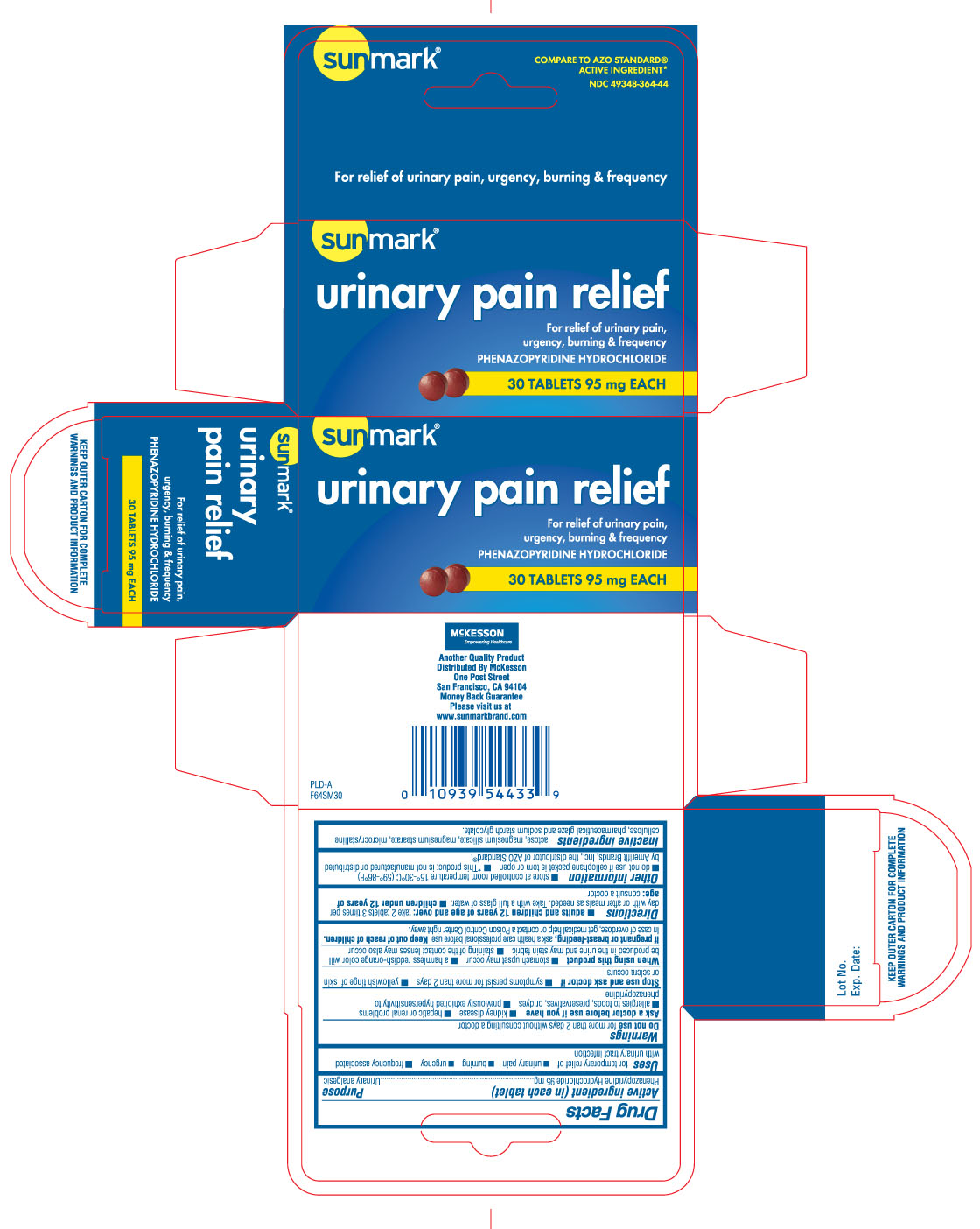
Phenazopyridine Hydrochloride 95 mg
| URINARY PAIN RELIEF
phenazopyridine hydrochloride tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Strategic Sourcing Services LLC (116956644) |