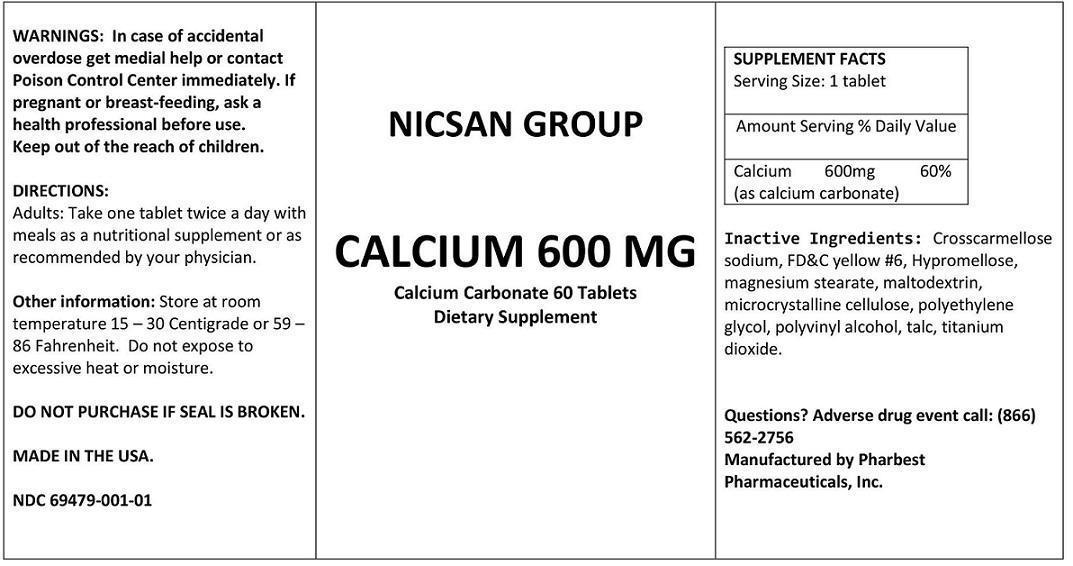
Label: CALCIUM 600 MG- calcium carbonate tablet
- NHRIC Code(s): 69479-001-01
- Packager: NICSAN GROUP INC
- Category: DIETARY SUPPLEMENT
- DEA Schedule: None
- Marketing Status: Dietary Supplement
Drug Label Information
Updated November 9, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Dietary Supplement
-
STATEMENT OF IDENTITY
SUPPLEMENT FACTS
Serving Size: 1 tabletAmount Serving % Daily Value Calcium
(as calcium carbonate)
600mg 60% Inactive Ingredients: Crosscarmellose sodium, FD&C yellow #6, Hypromellose, magnesium stearate,
maltodextrin, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, talc, titanium dioxide.
- WARNINGS
- PRECAUTIONS
- SAFE HANDLING WARNING
- DIRECTIONS:
- HEALTH CLAIM
- Packaging
-
INGREDIENTS AND APPEARANCE
CALCIUM 600 MG
calcium carbonate tabletProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:69479-001 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CATION 600 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) HYPROMELLOSES (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOLS (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL (UNII: 532B59J990) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:69479-001-01 60 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date dietary supplement 11/09/2015 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color shape size (solid drugs) 60 mm scoring 2 Labeler - NICSAN GROUP INC (079626857) Establishment Name Address ID/FEI Business Operations Pharbest Pharmaceuticals, Inc. 557054835 manufacture(69479-001)