PLUSPHARMA EXTRA STRENGTH PAIN RELIEVER,FEVER REDUCER 500 MG- acetaminophen tablet
Gemini Pharmaceuticals, Inc. dba Plus Pharma
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Acetaminophen 500 mg Caplet
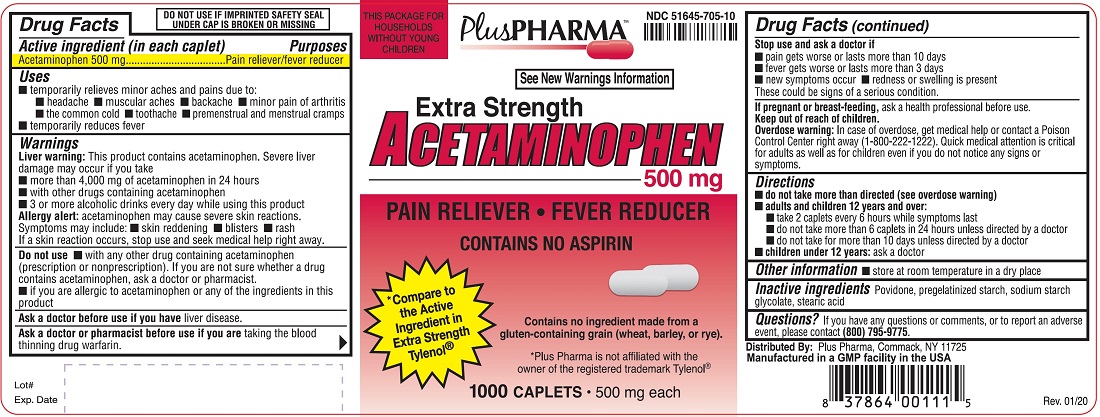
Uses
- temporarily relieves minor aches and pains due to:
- headache
- muscular aches
- backache
- minor pain of arthritis
- the common cold
- toothache
- premenstrual and menstrual cramps
- temporarily reduces fever
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert: acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or non prescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are allergic to acetaminophen or any of the inactive ingredients in this product
Directions
- do not take more than directed (see overdose warning)
- adults and children 12 years and over:
- take 2 caplets every 6 hours while symptoms last
- do not take more than 6 caplets in 24 hours unless directed by a doctor
- do not take for more than 10 days unless directed by a doctor
- children under 12 years: ask a doctor
Questions? If you have any questions or comments, or to report an adverse event, please contact (800) 795-9775.
1000-COUNT
THIS PACKAGE FOR HOUSEHOLDS WITHOUT YOUNG CHILDREN
PlusPHARMA™
NDC 51645-705-10
See New Warnings Information
Extra Strength
ACETAMINOPHEN 500 mg
PAIN RELIEVER • FEVER REDUCER
CONTAINS NO ASPIRIN
Compare to the Active Ingredient in Extra Strength Tylenol®
Contains no ingredient made from a gluten-containing grain (wheat, barley, or rye)
*Plus Pharma is not affiliated with the owner of the registered trademark Tylenol®
1000 CAPLETS • 500 mg each
100-COUNT
PlusPHARMA™
NDC 51645-705-01
See New Warnings Information
Extra Strength
ACETAMINOPHEN 500 mg
PAIN RELIEVER • FEVER REDUCER
CONTAINS NO ASPIRIN
Compare to the Active Ingredient in Extra Strength Tylenol®
Contains no ingredient made from a gluten-containing grain (wheat, barley, or rye)
*Plus Pharma is not affiliated with the owner of the registered trademark Tylenol®
100 CAPLETS • 500 mg each

| PLUSPHARMA EXTRA STRENGTH
PAIN RELIEVER,FEVER REDUCER 500 MG
acetaminophen tablet |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Gemini Pharmaceuticals, Inc. dba Plus Pharma (055942270) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Gemini Pharmaceuticals, Inc. dba Plus Pharma | 055942270 | manufacture(51645-705) | |