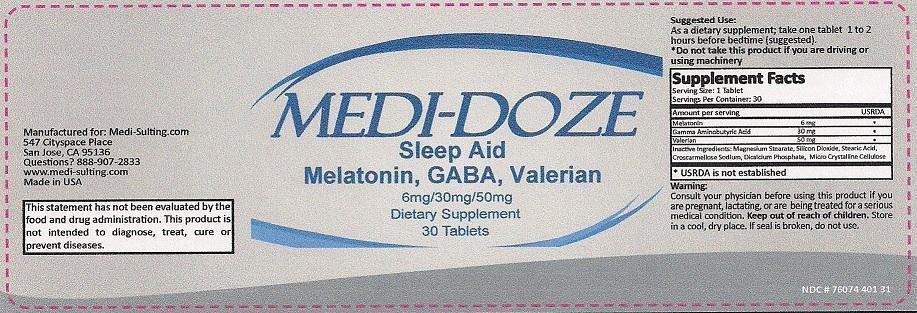
MEDI-DOZE RX SLEEP AID- melatonin, .gamma.-aminobutyric acid and valerian tablet
Two Hip Consulting, LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
WARNING:
CONSULT YOUR PHYSICIAN BEFORE USING THIS PRODUCT IF YOU ARE PREGNANT, LACTATING, OR ARE BEING TREATED FOR A SERIOUS MEDICAL CONDITION. KEEP OUT OF REACH OF CHILDREN.
| MEDI-DOZE RX
SLEEP AID
melatonin .gamma.-aminobutyric acid valerian tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Two Hip Consulting, LLC (965352896) |
| Registrant - Two Hip Consulting, LLC (965352896) |
Revised: 4/2016
Document Id: 250e5dec-62f3-47eb-8c5a-d71956890c0c
Set id: b450210c-516d-4068-b792-8bed31b26e32
Version: 3
Effective Time: 20160411
Two Hip Consulting, LLC