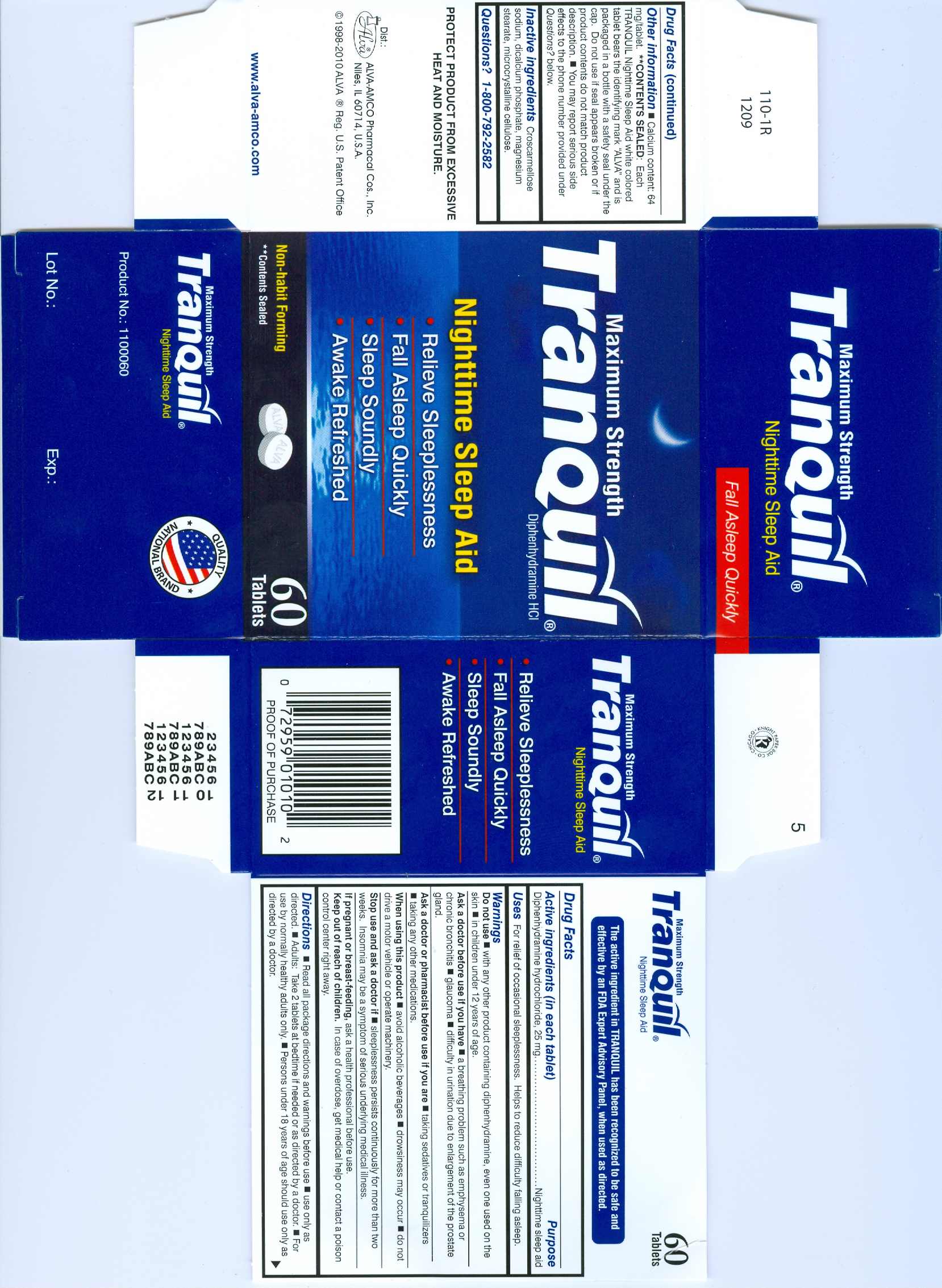
TRANQUIL NIGHTTIME- diphenhydramine hydrochloride tablet
Alva-Amco Pharmacal Companies, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Tranquil
Do not use
- with any other product containing diphenhydramine, even one used on the skin
- in children under 12 years of age
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urination due to enlargement of the prostate gland.
Ask a doctor or pharmacist before use if you are
- taking sedatives or tranquilizers
- taking any other medications.
When using this product
- avoid alcoholic beverages
- drowsiness may occur
- do not drive a motor vehicle or operate machinery
Stop use and ask a doctor if sleeplessness persists continuously for more than two weeks. Insomnia may be a symptom of serious underlying medical illness.
Keep out of reach of children. In case of overdose, get medical help or contact a poison control center right away.
Directions
- Read all package directions and warnings before use.
- Use only as directed.
- Adults: Take 2 tablets at bedtime if needed or as directed by a doctor.
- For use by normally healthy adults only.
- Persons under 18 years of age should use only as directed by a doctor.
Other information
- Calcium content: 64 mg/tablet.
- **Contents sealed: Each Tranquil Nighttime Sleep Aid white colored tablet bears the identifying mark "ALVA" and is packaged in a bottle with a safety seal under the cap. Do not use if seal appears broken or if product contents do not match product description.
- You may report serious side effects to the phone number provided under
Questions? below.
| TRANQUIL
NIGHTTIME
diphenhydramine hydrochloride tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Alva-Amco Pharmacal Companies, Inc. (042074856) |
Revised: 11/2018
Document Id: 79ee7397-ae58-fdbe-e053-2991aa0a0422
Set id: b392c7a1-2f89-4df2-806b-ad2e0a5ce995
Version: 3
Effective Time: 20181105
Alva-Amco Pharmacal Companies, Inc.