CONTROLRX MULTI- sodium fluoride paste, dentifrice
3M ESPE Dental Products
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
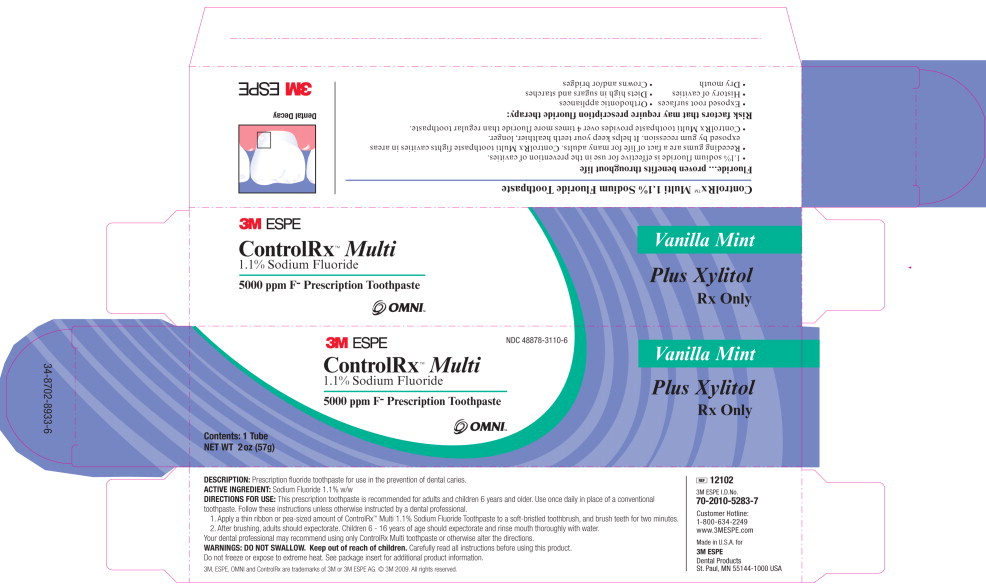
ControlRx™ Multi
1.1% Sodium Fluoride Perscription Toothpaste
DESCRIPTION:
ControlRx Multi dentifrice is a prescription formulation for use in the prevention of dental caries. This formulation contains 1.1% sodium fluoride in a neutral pH base and a mild abrasive to help remove dental plaque, debris and stain.
CLINICAL PHARMACOLOGY:
The use of higher-concentration fluoride products results in greater reductions in the incidence of dental caries. ControlRx Multi dentifrice provides enhanced remineralization of demineralized enamel and enhanced protection against subsequent acid challenges, relative to over the counter fluoride products.
INDICATIONS AND USAGE:
ControlRx Multi dentifrice is indicated for use as part of a professional program for the prevention and control of dental caries. ControlRx Multi dentifrice is applied to the teeth using a toothbrush. ControlRx Multi dentifrice should be used once daily in place of a conventional toothpaste, unless otherwise instructed by a dental professional.
CONTRAINDICATIONS:
Do not use in children less than 6 years of age unless recommended by a dental professional.
WARNINGS:
Do not swallow. Keep out of reach of children. Frequent ingestion may result in dental fluorosis in children less than 6 years of age, especially if community water fluoridation exceeds 0.6 ppm fluoride ion. Use in children less than 6 years of age requires special supervision to prevent swallowing. Carefully read all instructions before using this product.
OVERDOSAGE:
Medical attention should be sought if more than a thin ribbon or pea-sized amount is accidentally swallowed. A thinribbon or pea-sized amount of ControlRx Multi dentifrice weighs approximately 0.3g and contains approximately 1.5mg of fluoride ion. A 2 oz tube contains 282mg of fluoride ion.
DOSAGE AND ADMINISTRATION:
Follow these instructions unless otherwise instructed by a dental professional. Use once daily. Apply a thin ribbon or pea-sized amount of ControlRx Multi dentifrice to a soft-bristled toothbrush, and brush teeth for two minutes. After brushing, adults should expectorate. Children 6 to 16 years of age should expectorate and thoroughly rinse mouth with water.
| CONTROLRX MULTI
sodium fluoride paste, dentifrice |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - 3M ESPE Dental Products (801390852) |