Label: MVC-FLUORIDE- sodium fluoride, vitamin a, ascorbic acid, vitamin d, alpha-tocopherol, thiamine, riboflavin, niacin, pyridoxine, folic acid, and cyanocobalamin tablet, chewable
- NHRIC Code(s): 44946-1021-5
- Packager: Sancilio & Company Inc.
- Category: DIETARY SUPPLEMENT
- DEA Schedule: None
- Marketing Status: Dietary Supplement
Drug Label Information
Updated February 15, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
STATEMENT OF IDENTITY
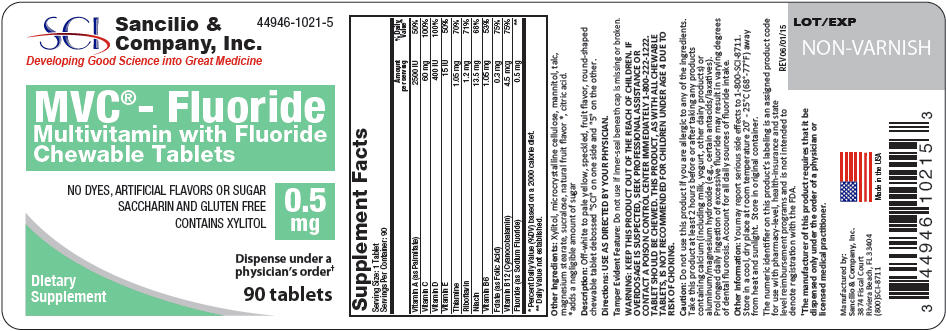
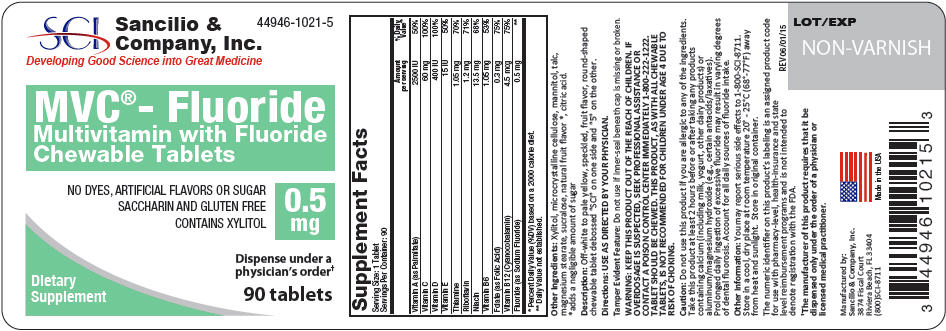
Each Multivitamin with 0.5 mg Fluoride Tablet Contains: - *
- Each Multivitamin with 0.5 mg Fluoride Tablet contains 0.5 mg Fluoride from 1.1 mg Sodium Fluoride (NaF).
Vitamin A (as Palmitate) 2500 IU Vitamin C 60 mg Vitamin D 400 IU Vitamin E 15 IU Thiamine 1.05 mg Riboflavin 1.2 mg Niacin 13.5 mg Vitamin B6 1.05 mg Folate (as Folic Acid) 0.3 mg Vitamin B12 4.5 mcg Fluoride (as Sodium Fluoride)* 0.5 mg -
WARNING
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN. IF OVERDOSAGE IS SUSPECTED, SEEK PROFESSIONAL ASSISTANCE OR CONTACT A POISON CONTROL CENTER IMMEDIATELY 1-800-222-1222. TABLET SHOULD BE CHEWED. THIS PRODUCT, AS WITH ALL CHEWABLE TABLETS, IS NOT RECOMMENDED FOR CHILDREN UNDER AGE 4 DUE TO RISK OF CHOKING.
DO NOT USE IF FOIL SEAL UNDER CAP IS BROKEN OR MISSING
- Directions
- Description
-
Caution
Do not use this product if you are allergic to any of the ingredients. Take this product at least 2 hours before or after taking any products containing calcium (including milk, yogurt, other dairy products) or aluminum/magnesium hydroxide (e.g., certain antacids/laxatives). Prolonged daily ingestion of excessive fluoride may result in varying degrees of dental fluorosis. Account for all daily sources of fluoride intake.
- Other Ingredients
- Storage
-
DOSAGE & ADMINISTRATION
This medical food product is formulated to be administered orally, under the ongoing supervision of a physician and is intended for the dietary management of dental caries for which a distinctive nutritional requirement of fluoride, based on recognized scientific principles, has been established by medical evaluation.
- HEALTH CLAIM
- PRINCIPAL DISPLAY PANEL - 0.5 mg Tablet Bottle Label
-
INGREDIENTS AND APPEARANCE
MVC-FLUORIDE
sodium fluoride, vitamin a, ascorbic acid, vitamin d, alpha-tocopherol, thiamine, riboflavin, niacin, pyridoxine, folic acid, and cyanocobalamin tablet, chewableProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:44946-1021 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Sodium Fluoride (UNII: 8ZYQ1474W7) (Fluoride Ion - UNII:Q80VPU408O) Fluoride Ion 0.5 mg Vitamin A (UNII: 81G40H8B0T) (Vitamin A - UNII:81G40H8B0T) Vitamin A 2500 [iU] Ascorbic Acid (UNII: PQ6CK8PD0R) (Ascorbic Acid - UNII:PQ6CK8PD0R) Ascorbic Acid 60 mg Vitamin D (UNII: 9VU1KI44GP) (Vitamin D - UNII:9VU1KI44GP) Vitamin D 400 [iU] .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) (.ALPHA.-TOCOPHEROL - UNII:H4N855PNZ1) .ALPHA.-TOCOPHEROL 15 [iU] Thiamine (UNII: X66NSO3N35) (THIAMINE ION - UNII:4ABT0J945J) Thiamine 1.05 mg Riboflavin (UNII: TLM2976OFR) (Riboflavin - UNII:TLM2976OFR) Riboflavin 1.2 mg Niacin (UNII: 2679MF687A) (Niacin - UNII:2679MF687A) Niacin 13.5 mg Pyridoxine (UNII: KV2JZ1BI6Z) (Pyridoxine - UNII:KV2JZ1BI6Z) Pyridoxine 1.05 mg Folic Acid (UNII: 935E97BOY8) (Folic Acid - UNII:935E97BOY8) Folic Acid 0.3 mg Cyanocobalamin (UNII: P6YC3EG204) (Cyanocobalamin - UNII:P6YC3EG204) Cyanocobalamin 4.5 ug Inactive Ingredients Ingredient Name Strength Xylitol (UNII: VCQ006KQ1E) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) Mannitol (UNII: 3OWL53L36A) Talc (UNII: 7SEV7J4R1U) Magnesium Stearate (UNII: 70097M6I30) Sucralose (UNII: 96K6UQ3ZD4) Anhydrous Citric Acid (UNII: XF417D3PSL) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:44946-1021-5 90 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Dietary Supplement 12/07/2011 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color scoring 1 shape size (solid drugs) 13 mm imprint flavor Labeler - Sancilio & Company Inc. (176681257) Establishment Name Address ID/FEI Business Operations Sancilio & Company Inc. 176681257 MANUFACTURE(44946-1021)