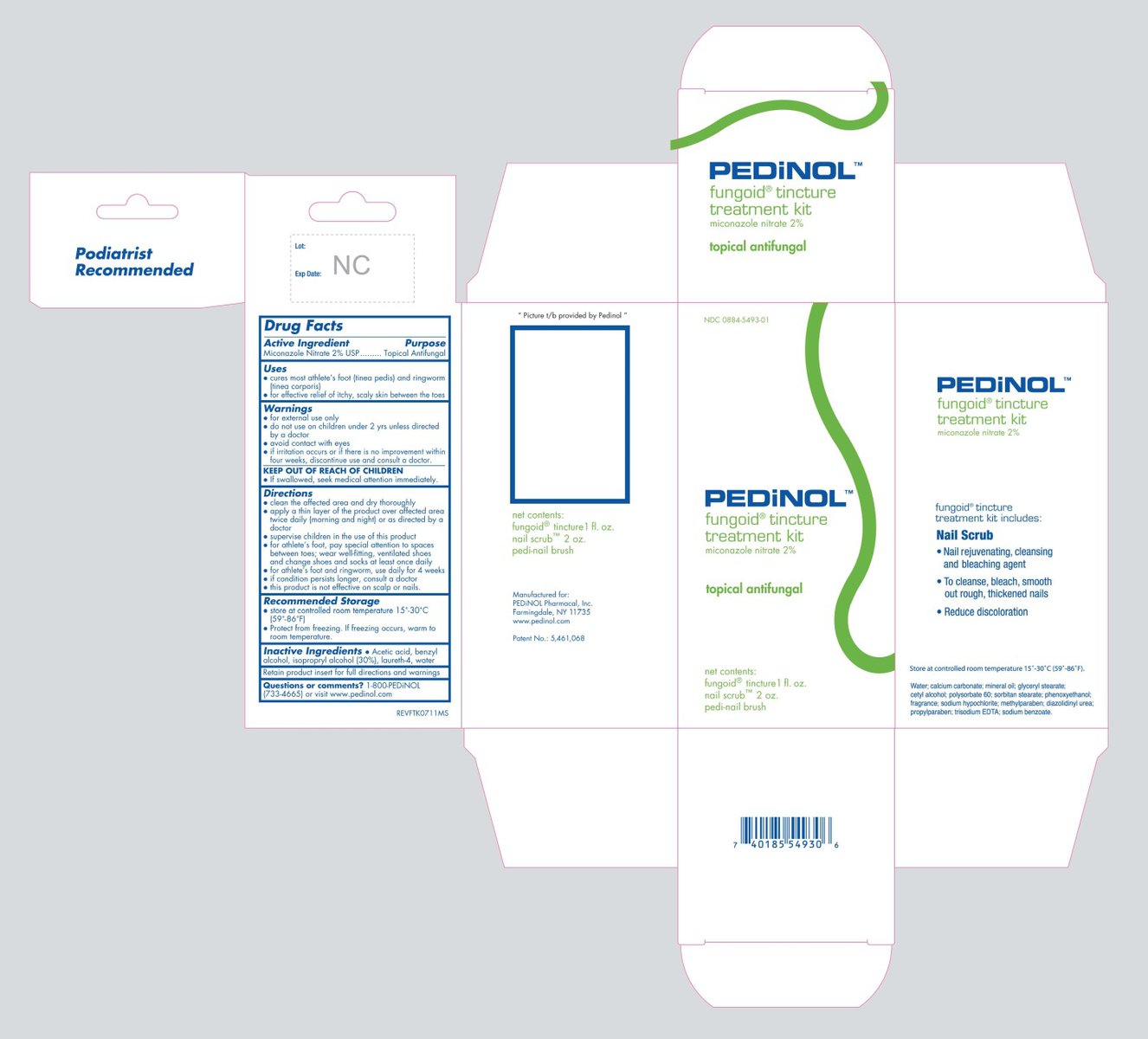
FUNGOID TINCTURE TREATMENT KIT- miconazole nitrate
Pedinol Pharmacal, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredient
Miconazole Nitrate 2%
Purpose
Topical Antifungal
Uses
- •
- cures most athlete's foot (tinea pedis) and ringworm (tinea corporis)
- •
- for effective relief of itchy, scaly skin between the toes
WARNINGS
- •
- for external use only.
- •
- do not use on children under 2 yrs unless directed by a doctor.
- •
- avoid contact with eyes.
- •
- if irritation occurs or if there is no improvement within four weeks, discontinue use and consult a doctor.
KEEP OUT OF REACH OF CHILDREN
If swallowed, seek medical attention immediately.
Directions
- •
- clean the affected area and dry thoroughly.
- •
- apply a thin layer of the product over affected area twice daily (morning and night) or as directed by a doctor
- •
- supervise children in the use of this product
- •
- for athlete's foot, pay special attention to spaces between toes; wear well-fitting, ventilated shoes and change shoes and socks at least once daily
- •
- for athlete’s foot and ringworm, use daily for 4 weeks
- •
- if condition persists longer, consult a doctor
- •
- this product is not effective on scalp or nails
Recommended Storage
- •
- store at controlled room temperature 15° - 30°C (59° - 86°F)
- •
- Protect from freezing. If freezing occurs, warm to room temperature.
Inactive Ingredients
Acetic acid, benzyl alcohol, isopropyl alcohol (30%), laureth-4, water
Retain product insert for full directions and warnings
Questions or comments?
1-800-PEDiNOL (733-4665) or visit www.pedinol.com
Principal Display Panel – Kit Carton
NDC 0884-5493-01
PEDiNOL™
fungoid® tincture
treatment kit
miconazole nitrate 2%
topical antifungal
net contents:
fungoid® tincture 1 fl. oz.
nail scrub™ 2 oz.
pedi-nail brush
Principal Display Panel – Nail Scrub Label
0884-4891-02
PEDiNOL™
nail scrub™
nail rejuvenating,
cleansing & bleaching
agent
net wt. 2 oz. (56.7g)
Pedinol Pharmacal, Inc.