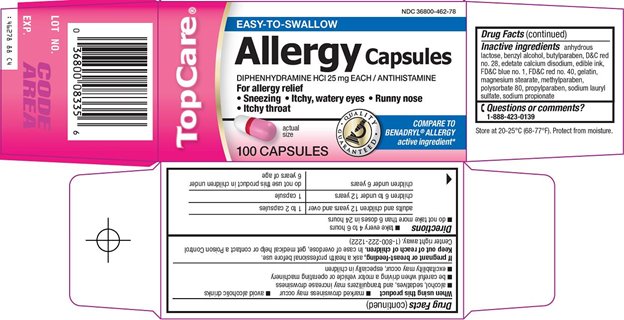
TOPCARE ALLERGY- diphenhydramine hydrochloride capsule
Topco Associates LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Topco Associates LLC. Allergy Capsules Drug Facts
Uses
- •
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- •
- runny nose
- •
- sneezing
- •
- itchy, watery eyes
- •
- itching of the nose or throat
- •
- temporarily relieves these symptoms due to the common cold:
- •
- runny nose
- •
- sneezing
Warnings
Do not use
- •
- with any other product containing diphenhydramine, even one used on skin
- •
- to make a child sleepy
Ask a doctor before use if you have
- •
- glaucoma
- •
- trouble urinating due to an enlarged prostate gland
- •
- a breathing problem such as emphysema or chronic bronchitis
Directions
- •
- take every 4 to 6 hours
- •
- do not take more than 6 doses in 24 hours
|
adults and children 12 years and over |
1 to 2 capsules |
|
children 6 to under 12 years |
1 capsule |
|
children under 6 years |
do not use this product in children under 6 years of age |
Inactive ingredients
anhydrous lactose, benzyl alcohol, butylparaben, D&C red no. 28, edetate calcium disodium, edible ink, FD&C blue no. 1, FD&C red no. 40, gelatin, magnesium stearate, methylparaben, polysorbate 80, propylparaben, sodium lauryl sulfate, sodium propionate
| TOPCARE ALLERGY
diphenhydramine hydrochloride capsule |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Topco Associates LLC (006935977) |
Revised: 11/2017
Document Id: d4c9ec61-60f6-4e82-a4e9-c4c08fb35050
Set id: b199a8c5-7345-4772-81c8-a965be0f5bd3
Version: 3
Effective Time: 20171110
Topco Associates LLC