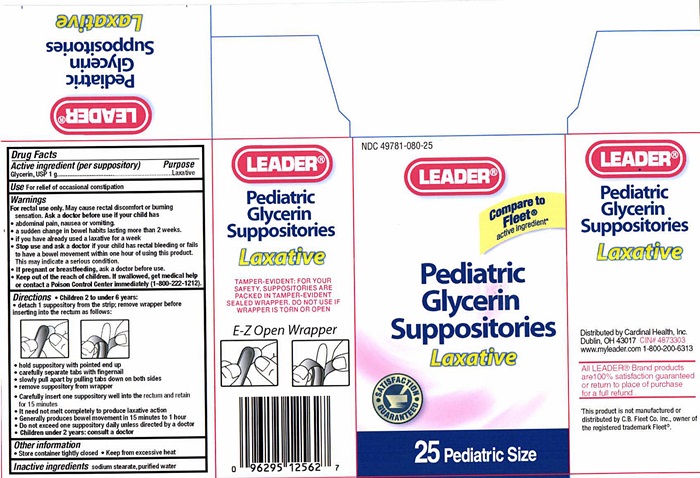
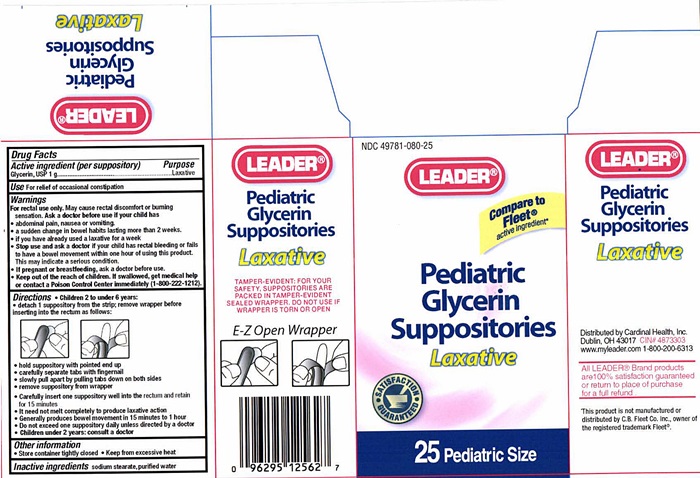
Label: PEDIATRIC GLYCERIN LAXATIVE- glycerin suppository
-
Contains inactivated NDC Code(s)
NDC Code(s): 49781-080-25 - Packager: Cardinal Health, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 19, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts Active ingredient
- Purpose
- Keep out of reach of children.
- Use
- Warnings
- Ask a doctor before use if your child has
- Stop use and ask a doctor if your child has
- If pregnant or breastfeeding,
-
Directions
- Children 2 to under 6 years:
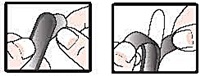
- detach 1 suppository from the strip; remove wrapper before inserting into the rectum as follows:
- hold suppository with pointed end up
- carefully separate tabs with fingernail
- slowly pull apart by pulling tabs down on both sides
- remove suppository from wrapper
- Carefully insert one suppository well into the rectum and retain for 15 minutes
- It need not melt completely to produce laxative action
- Generally produces bowel movement in 15 minutes to 1 hour
- Do not exceed one suppository daily unless directed by a doctor
- Children under 2 years: consult a doctor
- Inactive ingredients
- Other information
-
Pediatric Glycerin Suppository Label
NDC 49781-080-25
LEADER®
Compare to Fleet® active ingredient*
Pediatric Glycerin Suppositories
Laxative
SATISFACTION GUARANTEED
25 Pediatric Size
TAMPER-EVIDENT: FOR YOUR SAFETY , SUPPOSITORIES ARE PACKED IN TAMPER-EVIDENT SEALED WRAPPER. DO NOT USE IF WRAPPER IS TORN OR OPEN
E-Z Open Wrapper
Distributed by Cardinal Health, Inc.
Dublin, Ohio 43017
CIN 4873303
www.myleader.com
1-800-200-6313
All Leader® Brand products are 100% satisfaction guaranteed or return to place of purchase for a full refund.
This product is not manufactured or distributed by C.B. Fleet Co. Inc., owner of the registered trademark Fleet®.
-
INGREDIENTS AND APPEARANCE
PEDIATRIC GLYCERIN LAXATIVE
glycerin suppositoryProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49781-080 Route of Administration RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYCERIN (UNII: PDC6A3C0OX) (GLYCERIN - UNII:PDC6A3C0OX) GLYCERIN 1 g Inactive Ingredients Ingredient Name Strength SODIUM STEARATE (UNII: QU7E2XA9TG) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49781-080-25 25 in 1 BOX Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part334 04/01/2014 Labeler - Cardinal Health, Inc. (097537435) Registrant - Acino Products LLC (019385518) Establishment Name Address ID/FEI Business Operations Acino Products LLC 019385518 manufacture(49781-080)