FLUZONE INTRADERMAL QUADRIVALENT- influenza a virus a/california/7/2009 x-179a (h1n1) antigen (formaldehyde inactivated), influenza a virus a/switzerland/9715293/2013 nib-88 (h3n2) antigen (formaldehyde inactivated), influenza b virus b/phuket/3073/2013 antigen (formaldehyde inactivated), and influenza b virus b/brisbane/60/2008 antigen (formaldehyde inactivated) injection, suspension
Sanofi Pasteur Inc.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Fluzone® Intradermal Quadrivalent safely and effectively. See full prescribing information for Fluzone Intradermal Quadrivalent.
Fluzone Intradermal Quadrivalent (Influenza Vaccine) Suspension for Intradermal Injection 2015-2016 Formula Initial U.S. Approval (Fluzone Intradermal Quadrivalent): 2014 INDICATIONS AND USAGEFluzone Intradermal Quadrivalent is a vaccine indicated for active immunization for the prevention of influenza disease caused by influenza A subtype viruses and type B viruses contained in the vaccine. (1) Fluzone Intradermal Quadrivalent is approved for use in persons 18 through 64 years of age. (1) DOSAGE AND ADMINISTRATIONDOSAGE FORMS AND STRENGTHSSuspension for injection in a prefilled microinjection system, 0.1 mL. (3) CONTRAINDICATIONSSevere allergic reaction (e.g., anaphylaxis) to any component of the vaccine, including egg protein, or after previous dose of any influenza vaccine. (4) WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
To report SUSPECTED ADVERSE REACTIONS, contact Sanofi Pasteur Inc., at 1-800-822-2463 (1-800-VACCINE) or Vaccine Adverse Event Reporting System (VAERS) at 1-800-822-7967 or www.vaers.hhs.gov. USE IN SPECIFIC POPULATIONSSee 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 6/2015 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
Fluzone® Intradermal Quadrivalent is indicated for active immunization for the prevention of influenza disease caused by influenza A subtype viruses and type B viruses contained in the vaccine.
Fluzone Intradermal Quadrivalent is approved for use in persons 18 through 64 years of age.
2 DOSAGE AND ADMINISTRATION
- For intradermal use only
2.1 Dose and Schedule
Fluzone Intradermal Quadrivalent should be administered as a single 0.1 mL injection by the intradermal route in adults 18 through 64 years of age.
2.2 Administration
Inspect Fluzone Intradermal Quadrivalent visually for particulate matter and/or discoloration prior to administration. If either of these conditions exist, the vaccine should not be administered.
The preferred site of injection is the skin in the region of the deltoid. Note: A potential way to make vaccination more efficient is to have the patient place the hand of the arm being immunized on his/her hip, so the arm bends at the elbow. This can help create a more accessible angle to the skin in the deltoid region.
Fluzone Intradermal Quadrivalent should not be combined through reconstitution or mixed with any other vaccine.
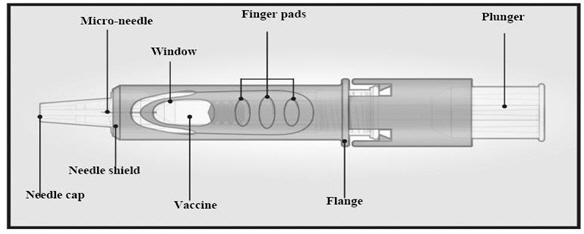
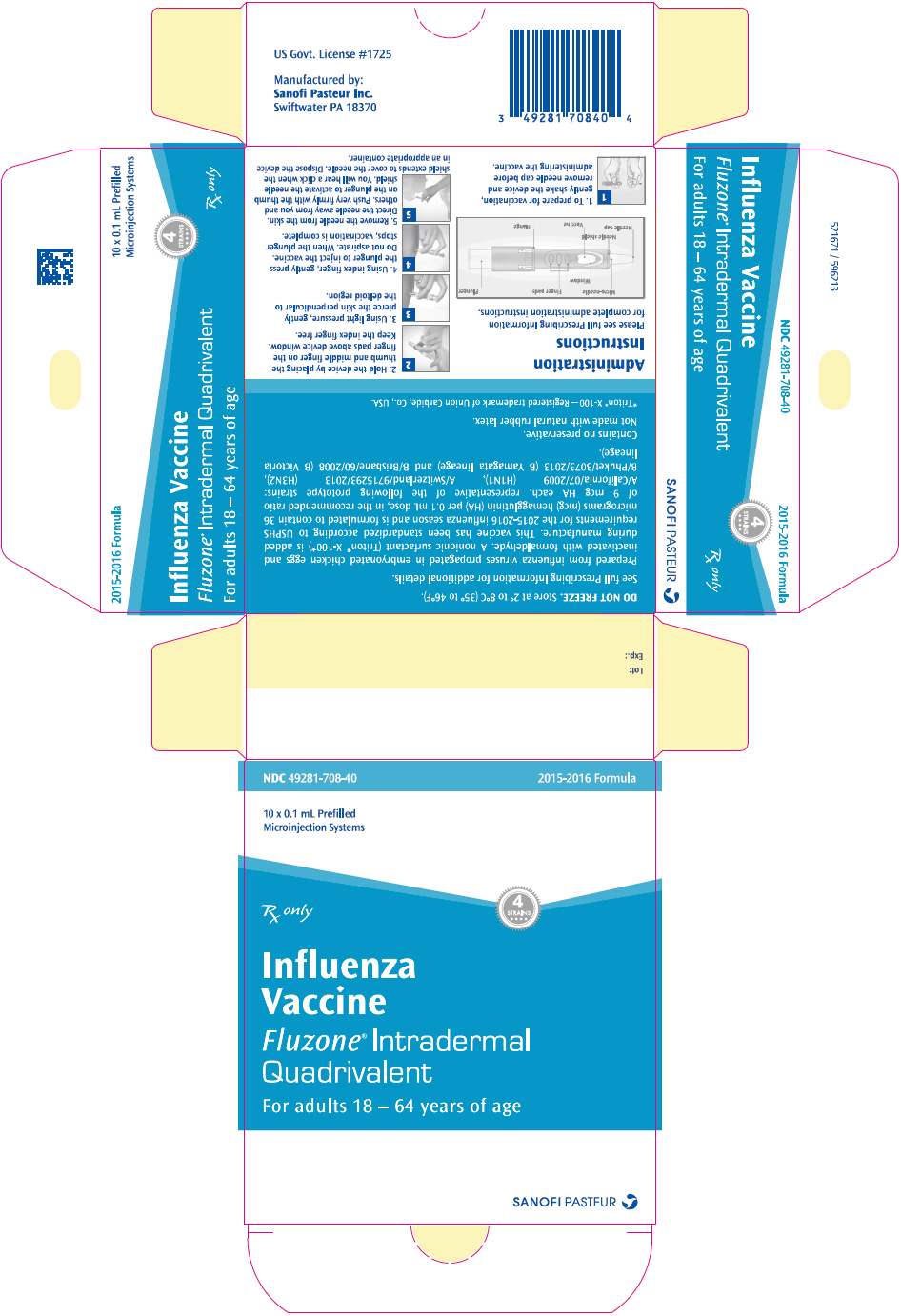
- 1.
-
Gently shake the device and remove needle cap
To prepare for vaccination, gently shake the device and remove needle cap before administering the vaccine.
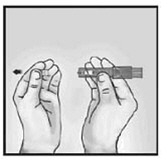
- 2.
-
Position the device in your hand between the thumb and middle finger, keeping the index finger free
Hold the device by placing the thumb and middle finger on the finger pads above device window. Keep the index finger free.
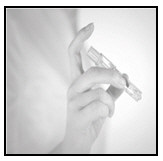
- 3.
-
Gently pierce the skin over the deltoid region
Using light pressure, gently pierce the skin perpendicular to the deltoid region.
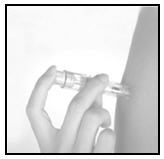
- 4.
-
Press the plunger to inject the vaccine
Using index finger, gently press the plunger to inject the vaccine. Do not aspirate. When the plunger stops, vaccination is complete. Note: Excessive pressure on the plunger may prematurely activate the needle shield on the patient's arm. Because the vaccine is injected into the skin, a wheal (superficial bump) and/or redness may be visible at the injection site.

- 5.
-
Activate the needle shield and dispose
Remove the needle from the skin. Direct the needle away from you and others. Push very firmly with the thumb on the plunger to activate the needle shield. You will hear a click when the shield extends to cover the needle. Dispose the device in an appropriate container.
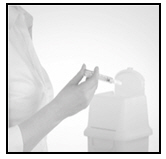
3 DOSAGE FORMS AND STRENGTHS
Fluzone Intradermal Quadrivalent is a suspension for injection.
Fluzone Intradermal Quadrivalent is supplied in a single-dose prefilled microinjection system, 0.1 mL, for adults 18 through 64 years of age.
4 CONTRAINDICATIONS
Do not administer Fluzone Intradermal Quadrivalent to anyone with a history of a severe allergic reaction (e.g., anaphylaxis) to any component of the vaccine [see Description (11)], including egg protein, or to a previous dose of any influenza vaccine.
5 WARNINGS AND PRECAUTIONS
5.1 Guillain-Barré Syndrome
If Guillain-Barré syndrome (GBS) has occurred within 6 weeks following previous influenza vaccination, the decision to give Fluzone Intradermal Quadrivalent should be based on careful consideration of the potential benefits and risks. The 1976 swine influenza vaccine was associated with an elevated risk of GBS. Evidence for a causal relation of GBS with other influenza vaccines is inconclusive; if an excess risk exists, it is probably slightly more than 1 additional case per 1 million persons vaccinated (See references 1 and 2).
5.2 Preventing and Managing Allergic Reactions
Appropriate medical treatment and supervision must be available to manage possible anaphylactic reactions following administration of Fluzone Intradermal Quadrivalent.
6 ADVERSE REACTIONS
In adults 18 through 64 years of age, the most common (≥10%) injection-site reactions were pain (53.3%), pruritus (52.1%), erythema (36.7%), swelling (19.5%), and induration (17.0%); the most common solicited systemic adverse reactions were myalgia (34.1%), headache (33.1%), malaise (27.7%), and shivering (12.1%).
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse event rates observed in the clinical trial(s) of a vaccine cannot be directly compared to rates in the clinical trial(s) of another vaccine, and may not reflect the rates observed in practice.
Fluzone Intradermal Quadrivalent in Adults 18 Through 64 Years of Age
Study 1 (NCT01712984, see http://clinicaltrials.gov) was a randomized, double-blind, active-controlled, multi-center safety and immunogenicity study conducted in the US. In this study, adults 18 through 64 years of age received a single injection of either Fluzone Intradermal Quadrivalent or one of two formulations of a comparator trivalent influenza vaccine by the intradermal route (TIV-ID1 or TIV-ID2). Each of the trivalent formulations contained an influenza type B virus that corresponded to one of the two type B viruses in Fluzone Intradermal Quadrivalent (a type B virus of the Victoria lineage or a type B virus of the Yamagata lineage). The safety analysis set, comprised of all participants who received a study vaccine, included 3355 recipients. Among participants in the three vaccine groups combined, 61.3% were female, 84.9% White, 11.9% Black, 1.1% Asian, and 2.1% were of other racial/ethnic groups. Table 1 summarizes solicited injection-site and systemic adverse reactions reported within 7 days post-vaccination via diary cards. Participants were monitored for unsolicited adverse events for 28 days after vaccination and serious adverse events (SAEs) for 6 months after vaccination.
| Fluzone Intradermal Quadrivalent (N‡=1649-1656) | TIV-ID1§
(B Yamagata) (N‡=819-820) | TIV-ID2¶
(B Victoria) (N‡=836-838) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Any (%) | Grade 2#
(%) | Grade 3Þ
(%) | Any (%) | Grade 2#
(%) | Grade 3Þ
(%) | Any (%) | Grade 2#
(%) | Grade 3Þ
(%) |
|
|
|||||||||
| Injection-site adverse reactions | |||||||||
| Pain | 53.3 | 9.7 | 1.4 | 48.2 | 7.9 | 1.2 | 50.1 | 7.5 | 1.4 |
| Pruritis | 52.1 | 9.4 | 2.8 | 45.4 | 9.0 | 1.8 | 44.6 | 7.5 | 2.3 |
| Erythema | 36.7 | 10.9 | 0.4 | 34.0 | 9.8 | 0.1 | 32.1 | 6.3 | 0.4 |
| Swelling | 19.5 | 4.8 | 0.1 | 14.8 | 3.9 | 0.0 | 14.7 | 2.0 | 0.0 |
| Induration | 17.0 | 2.8 | <0.1 | 13.5 | 1.8 | 0.0 | 11.2 | 2.2 | 0.0 |
| Ecchymosis | 2.6 | 0.4 | 0.0 | 1.8 | 0.4 | 0.0 | 1.8 | 0.1 | 0.0 |
| Systemic adverse reactions | |||||||||
| Myalgia | 34.1 | 8.1 | 2.6 | 29.0 | 8.2 | 1.5 | 31.1 | 7.4 | 2.5 |
| Headache | 33.1 | 9.1 | 3.2 | 31.3 | 9.6 | 2.4 | 33.2 | 8.9 | 1.8 |
| Malaise | 27.7 | 9.2 | 3.0 | 26.3 | 6.6 | 1.8 | 30.4 | 8.4 | 2.5 |
| Shivering | 12.1 | 2.0 | 1.4 | 10.4 | 2.2 | 0.6 | 11.2 | 3.3 | 1.6 |
| Fever (≥100.4°F)ß | 0.8 | 0.2 | 0.2 | 0.7 | 0.2 | 0.1 | 0.5 | 0.0 | 0.0 |
Unsolicited non-serious adverse events were reported in 382 (22.8%) recipients in the Fluzone Intradermal Quadrivalent group, 169 (20.2%) recipients in the TIV-ID1 group, and 212 (25.1%) recipients in the TIV-ID2 group. The most commonly reported unsolicited non-serious adverse events were cough, headache, and oropharyngeal pain. During the 28 days following vaccination, a total of 6 (0.4%) recipients in the Fluzone Intradermal Quadrivalent group, 2 (0.2%) recipients in the TIV-ID1 group, and 3 (0.4%) recipients in the TIV-ID2 group experienced at least one SAE; no deaths occurred. Throughout the study period (6 months post-vaccination), a total of 20 (1.2%) recipients in the Fluzone Intradermal Quadrivalent group, 14 (1.7%) recipients in the TIV-ID1 group, and 11 (1.3%) recipients in the TIV-ID2 group experienced at least one SAE. One death (177 days post-vaccination due to acute coronary myocardial infarction) occurred in the Fluzone Intradermal Quadrivalent group. This death was considered not related to the study vaccine by the Investigator.
Fluzone Intradermal (Trivalent Influenza Vaccine) in Adults 18 Through 64 Years of Age
The safety experience with Fluzone Intradermal (trivalent influenza vaccine) is relevant to Fluzone Intradermal Quadrivalent because both vaccines are manufactured using the same process and have overlapping compositions. In a study of adults 18 through 64 years of age (NCT00772109), safety was evaluated in 2855 Fluzone Intradermal recipients compared to 1421 Fluzone (trivalent influenza vaccine) recipients. Rates of solicited injection-site reactions and systemic adverse events in adults are shown in Table 2.
| Fluzone Intradermal (N*=2798-2802) Percentage | Fluzone (N*=1392-1394) Percentage |
|||||
|---|---|---|---|---|---|---|
| Any | Grade 2† | Grade 3‡ | Any | Grade 2† | Grade 3‡ | |
|
||||||
| Injection-Site Erythema | 76.4 | 28.8 | 13.0 | 13.2 | 2.1 | 0.9 |
| Injection-Site Induration | 58.4 | 13.0 | 3.4 | 10.0 | 2.3 | 0.5 |
| Injection-Site Swelling | 56.8 | 13.4 | 5.4 | 8.4 | 2.1 | 0.9 |
| Injection-Site Pain | 51.0 | 4.4 | 0.6 | 53.7 | 5.8 | 0.8 |
| Injection-Site Pruritus | 46.9 | 4.1 | 1.1 | 9.3 | 0.4 | 0.0 |
| Injection-Site Ecchymosis | 9.3 | 1.4 | 0.4 | 6.2 | 1.1 | 0.4 |
| Headache | 31.2 | 6.4 | 1.5 | 30.3 | 6.5 | 1.6 |
| Myalgia | 26.5 | 4.6 | 1.5 | 30.8 | 5.5 | 1.4 |
| Malaise | 23.3 | 5.5 | 2.2 | 22.2 | 5.5 | 1.8 |
| Shivering | 7.3 | 1.5 | 0.7 | 6.2 | 1.1 | 0.6 |
| Fever§ (≥99.5ºF) | 3.9 | 0.6 | 0.1 | 2.6 | 0.4 | 0.2 |
6.2 Post-Marketing Experience
Currently, there are no post-marketing data available for Fluzone Intradermal Quadrivalent.
The following events have been spontaneously reported during the post-approval use of the trivalent formulation of Fluzone. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to vaccine exposure. Adverse events were included based on one or more of the following factors: severity, frequency of reporting, or strength of evidence for a causal relationship to Fluzone.
- Blood and Lymphatic System Disorders: Thrombocytopenia, lymphadenopathy
- Immune System Disorders: Anaphylaxis, other allergic/hypersensitivity reactions (including urticaria, angioedema)
- Eye disorders: Ocular hyperemia
- Nervous System Disorders: Guillain-Barré syndrome (GBS), convulsions, febrile convulsions, myelitis (including encephalomyelitis and transverse myelitis), facial palsy (Bell's palsy), optic neuritis/neuropathy, brachial neuritis, syncope (shortly after vaccination), dizziness, paresthesia
- Vascular Disorders: Vasculitis, vasodilation/flushing
- Respiratory, Thoracic and Mediastinal Disorders: Dyspnea, pharyngitis, rhinitis, cough, wheezing, throat tightness
- Skin and Subcutaneous Tissue Disorders: Stevens-Johnson syndrome
- General Disorders and Administration Site Conditions: Pruritus, asthenia/fatigue, pain in extremities, chest pain
- Gastrointestinal Disorders: Vomiting
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B: The developmental and reproductive toxicity study performed with the trivalent formulation of Fluzone Intradermal is relevant to Fluzone Intradermal Quadrivalent because both vaccines share the same manufacturing process and route of administration. The study, in which the trivalent formulation of Fluzone Intradermal (27 mcg) was administered to female rabbits at a dose approximately 20 times the human dose (on a mg/kg basis), revealed no evidence of impaired female fertility or harm to the fetus. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, Fluzone Intradermal Quadrivalent should be used during pregnancy only if clearly needed.
Sanofi Pasteur Inc. is maintaining a prospective pregnancy exposure registry to collect data on pregnancy outcomes and newborn health status following vaccination with Fluzone Intradermal Quadrivalent during pregnancy. Healthcare providers are encouraged to enroll women who receive Fluzone Intradermal Quadrivalent during pregnancy in Sanofi Pasteur Inc.'s vaccination pregnancy registry by calling 1-800-822-2463.
8.3 Nursing Mothers
It is not known whether Fluzone Intradermal Quadrivalent is excreted in human milk. Because many drugs are excreted in human milk, the decision to give Fluzone Intradermal Quadrivalent to a nursing woman should be based on careful consideration of the potential benefits and risks.
8.4 Pediatric Use
Safety and effectiveness of Fluzone Intradermal Quadrivalent in persons <18 years of age have not been established. In a clinical trial, 97 infants and toddlers 6 months through 35 months of age and 160 children 3 years through 8 years of age were enrolled to receive two injections of the trivalent formulation of Fluzone Intradermal. Infants and children in a control group received two injections of Fluzone. Fluzone Intradermal was associated with increased local reactogenicity relative to Fluzone. The size of the study was not adequate to reliably evaluate serious adverse events or the immune response elicited by Fluzone Intradermal relative to Fluzone.
11 DESCRIPTION
Fluzone Intradermal Quadrivalent (Influenza Vaccine) for intradermal injection is an inactivated influenza vaccine, prepared from influenza viruses propagated in embryonated chicken eggs. The virus-containing allantoic fluid is harvested and inactivated with formaldehyde. Influenza virus is concentrated and purified in a linear sucrose density gradient solution using a continuous flow centrifuge. The virus is then chemically disrupted using a non-ionic surfactant, octylphenol ethoxylate (Triton® X-100), producing a "split virus". The split virus is further purified and then suspended in sodium phosphate-buffered isotonic sodium chloride solution. The Fluzone Intradermal Quadrivalent process uses an additional concentration factor after the ultrafiltration step in order to obtain a higher hemagglutinin (HA) antigen concentration. Antigens from the four strains included in the vaccine are produced separately and then combined to make the quadrivalent formulation.
Fluzone Intradermal Quadrivalent suspension for injection is clear and slightly opalescent in color.
Neither antibiotics nor preservative are used in the manufacture of Fluzone Intradermal Quadrivalent.
The Fluzone Intradermal Quadrivalent microinjection system is not made with natural rubber latex.
Fluzone Intradermal Quadrivalent is standardized according to United States Public Health Service requirements and is formulated to contain the following four influenza strains recommended for the 2015-2016 influenza season: A/California/07/2009 X-179A (H1N1), A/Switzerland/9715293/2013 NIB-88 (H3N2), B/Phuket/3073/2013 (B Yamagata lineage), and B/Brisbane/60/2008 (B Victoria lineage). The amounts of HA and other ingredients per dose of vaccine are listed in Table 3.
| Ingredient | Quantity per 0.1 mL Dose |
|---|---|
| Active Substance: Split influenza virus, inactivated strains*: | 36 mcg HA total |
| A (H1N1) | 9 mcg HA |
| A (H3N2) | 9 mcg HA |
| B/(Victoria lineage) | 9 mcg HA |
| B/(Yamagata lineage) | 9 mcg HA |
| Other: | |
| Sodium phosphate-buffered isotonic sodium chloride solution | QS† to appropriate volume |
| Formaldehyde | ≤20 mcg |
| Octylphenol ethoxylate | ≤55 mcg |
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Influenza illness and its complications follow infection with influenza viruses. Global surveillance of influenza identifies yearly antigenic variants. Since 1977, antigenic variants of influenza A (H1N1 and H3N2) viruses and influenza B viruses have been in global circulation. Since 2001, two distinct lineages of influenza B (Victoria and Yamagata lineages) have co-circulated worldwide. Protection from influenza virus infection has not been correlated with a specific level of hemagglutination inhibition (HAI) antibody titer post-vaccination. However, in some human studies, antibody titers ≥1:40 have been associated with protection from influenza illness in up to 50% of subjects (See references 3 and 4).
Antibodies against one influenza virus type or subtype confer limited or no protection against another. Furthermore, antibodies to one antigenic variant of influenza virus might not protect against a new antigenic variant of the same type or subtype. Frequent development of antigenic variants through antigenic drift is the virologic basis for seasonal epidemics and the reason for the usual change of one or more new strains in each year's influenza vaccine. Therefore, influenza vaccines are standardized to contain the hemagglutinins of influenza virus strains, representing the influenza viruses likely to be circulating in the US during the influenza season.
Annual vaccination with the current vaccine is recommended because immunity during the year after vaccination declines, and because circulating strains of influenza virus change from year to year.
14 CLINICAL STUDIES
14.1 Immunogenicity of Fluzone Intradermal Quadrivalent in Adults 18 through 64 Years of Age
In Study 1 (NCT01712984), study participants were randomized to receive one dose of Fluzone Intradermal Quadrivalent, TIV-ID1, or TIV-ID2. Of the 2249 participants randomized to provide blood samples for immunogenicity analyses, 2113 adults 18 through 64 years of age were included in the per-protocol analysis set. The distribution of demographics was similar to that of the safety analysis set [see Clinical Trials Experience (6.1)].
HAI antibody geometric mean titers (GMTs) and seroconversion rates 28 days following vaccination with Fluzone Intradermal Quadrivalent were non-inferior to those following each TIV-ID for all four strains, based on pre-specified criteria (see Table 4 and Table 5).
|
|||||||
| Antigen Strain | Fluzone Intradermal Quadrivalent | Pooled TIV-ID‡ | GMT Ratio (95% CI)§ |
||||
| M¶ | GMT | M¶ | GMT | ||||
| A (H1N1) | 1041 | 589 | 1072 | 680 | 0.87 (0.78; 0.97) | ||
| A (H3N2) | 1041 | 368 | 1071 | 430 | 0.86 (0.77; 0.96) | ||
| Fluzone Intradermal Quadrivalent | TIV-ID1#
(B Yamagata) | TIV-ID2Þ
(B Victoria) | GMT Ratio (95% CI)§ |
||||
| M¶ | GMT | M¶ | GMT | M¶ | GMT | ||
| B/Texas/6/2011 (B Yamagata) | 1041 | 105 | 539 | 93.5 | 533 | (54.0)ß | 1.13 (1.02; 1.25) |
| B/Brisbane/60/2008 (B Victoria) | 1041 | 136 | 538 | (66.7)à | 533 | 130 | 1.05 (0.94; 1.16) |
|
|||||||
| Antigen Strain | Fluzone Intradermal Quadrivalent | Pooled TIV-ID‡ | Difference of Seroconversion Rates (95% CI)§ |
||||
| M¶ | Seroconversion# (%) | M¶ | Seroconversion# (%) | ||||
| A (H1N1) | 1041 | 57.6 | 1072 | 60.4 | -2.72 (-6.90; 1.47) | ||
| A (H3N2) | 1040 | 58.5 | 1071 | 59.8 | -1.30 (-5.48; 2.89) | ||
| Fluzone Intradermal Quadrivalent | TIV-ID1Þ
(B Yamagata) | TIV-ID2ß
(B Victoria) | Difference of Seroconversion Rates (95% CI)§ |
||||
| M¶ | Seroconversion# (%) | M¶ | Seroconversion# (%) | M¶ | Seroconversion# (%) | ||
| B/Texas/6/2011 (B Yamagata) | 1041 | 55.7 | 539 | 46.9 | 533 | (24.6)à | 8.78 (3.58; 13.9) |
| B/Brisbane/60/2008 (B Victoria) | 1041 | 50.4 | 538 | (22.1)è | 533 | 44.1 | 6.34 (1.13; 11.5) |
15 REFERENCES
- 1
- Lasky T, Terracciano GJ, Magder L, et al. The Guillain-Barré syndrome and the 1992-1993 and 1993-1994 influenza vaccines. N Engl J Med 1998;339:1797-802.
- 2
- Baxter, R, et al. Lack of Association of Guillain-Barré Syndrome with Vaccinations. Clin Infect Dis 2013;57(2):197-204.
- 3
- Hannoun C, Megas F, Piercy J. Immunogenicity and protective efficacy of influenza vaccination. Virus Res 2004;103:133-138.
- 4
- Hobson D, Curry RL, Beare AS, Ward-Gardner A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg Camb 1972;70:767-777.
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Information). Inform the vaccine recipient or guardian:
- Fluzone Intradermal Quadrivalent contains killed viruses and cannot cause influenza.
- Fluzone Intradermal Quadrivalent stimulates the immune system to protect against influenza, but does not prevent other respiratory infections.
- Annual influenza vaccination is recommended.
- Because the vaccine is injected into the skin, patients may experience visible reactions at the injection site, such as a wheal (superficial bump), redness, and swelling. Patients may also experience pain, itching, and induration at the injection site.
- Report adverse reactions to their healthcare provider and/or to the Vaccine Adverse Event Reporting System (VAERS) at 1-800-822-7967 or http://vaers.hhs.gov.
- Sanofi Pasteur Inc. is maintaining a prospective pregnancy exposure registry to collect data on pregnancy outcomes and newborn health status following vaccination with Fluzone Intradermal Quadrivalent during pregnancy. Women who receive Fluzone Intradermal Quadrivalent during pregnancy are encouraged to contact Sanofi Pasteur Inc. directly or have their healthcare provider contact Sanofi Pasteur Inc. at 1-800-822-2463.
Fluzone is a registered trademark of Sanofi Pasteur Inc.
Patient Information Sheet
Fluzone® Intradermal Quadrivalent
Influenza Vaccine
Please read this information sheet before getting Fluzone Intradermal Quadrivalent vaccine. This summary is not intended to take the place of talking with your healthcare provider. If you have questions or would like more information, please talk with your healthcare provider.
What is Fluzone Intradermal Quadrivalent vaccine?
Fluzone Intradermal Quadrivalent is a vaccine that helps protect against influenza illness (flu).
Fluzone Intradermal Quadrivalent vaccine is for people who are 18 through 64 years of age.
Vaccination with Fluzone Intradermal Quadrivalent vaccine may not protect all people who receive the vaccine.
Who should not get Fluzone Intradermal Quadrivalent vaccine?
You should not get Fluzone Intradermal Quadrivalent vaccine if you:
- ever had a severe allergic reaction to eggs or egg products.
- ever had a severe allergic reaction after getting any flu vaccine.
- are younger than 18 years of age.
- are 65 years of age or older.
Tell your healthcare provider if you have or have had:
- Guillain-Barré syndrome (severe muscle weakness) after getting a flu vaccine.
- problems with your immune system as the immune response may be diminished.
How is the Fluzone Intradermal Quadrivalent vaccine given?
Fluzone Intradermal Quadrivalent vaccine is a shot given into the skin of the arm.
What are the possible side effects of Fluzone Intradermal Quadrivalent vaccine?
The most common side effects of Fluzone Intradermal Quadrivalent vaccine are:
- pain, redness, swelling, hardness, and itching where you got the shot
- muscle ache
- tiredness
- headache
- shivering
Once the vaccine has been given, a bump may be visible where you got the shot. These are not all of the possible side effects of Fluzone Intradermal Quadrivalent vaccine. You can ask your healthcare provider for a list of other side effects that is available to healthcare professionals.
Call your healthcare provider for advice about any side effects that concern you. You may report side effects to the Vaccine Adverse Event Reporting System (VAERS) at 1-800-822-7967 or http://vaers.hhs.gov.
Sanofi Pasteur Inc. is collecting information on pregnancy outcomes and the health of newborns following vaccination with Fluzone Intradermal Quadrivalent vaccine during pregnancy. Women who receive Fluzone Intradermal Quadrivalent vaccine during pregnancy are encouraged to contact Sanofi Pasteur Inc. directly or have their healthcare provider contact Sanofi Pasteur Inc. at 1-800-822-2463.
What are the ingredients in Fluzone Intradermal Quadrivalent vaccine?
Fluzone Intradermal Quadrivalent vaccine contains 4 killed flu virus strains.
Inactive ingredients include formaldehyde and octylphenol ethoxylate.
Manufactured by:
Sanofi Pasteur Inc.
Swiftwater, PA 18370 USA
| FLUZONE INTRADERMAL QUADRIVALENT
influenza a virus a/california/7/2009 x-179a (h1n1) antigen (formaldehyde inactivated), influenza a virus a/switzerland/9715293/2013 nib-88 (h3n2) antigen (formaldehyde inactivated), influenza b virus b/phuket/3073/2013 antigen (formaldehyde inactivated), and influenza b virus b/brisbane/60/2008 antigen (formaldehyde inactivated) injection, suspension |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Sanofi Pasteur Inc. (086723285) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sanofi Pasteur Inc. | 086723285 | MANUFACTURE | |