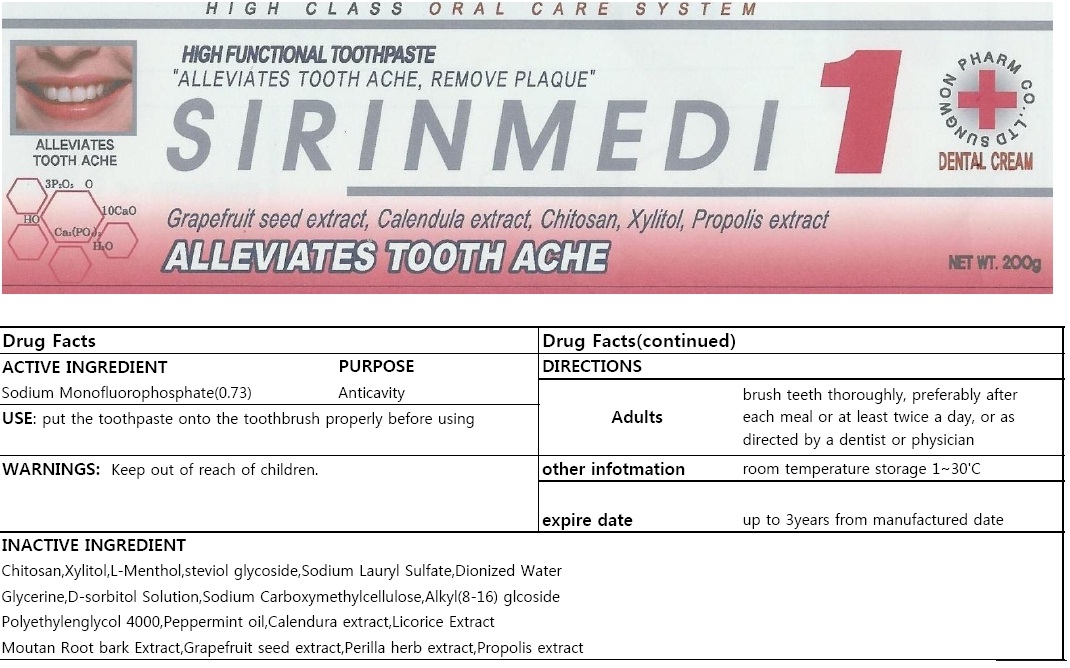
SIRINMEDI ONE- sodium monofluorophosphate paste, dentifrice
Sungwon Pharmaceutical Co., Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Warning
If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
Direction
| adults and children 2 years of age and older | brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or physician |
| children 2 to 6 years | use only a pea sized amount and supervise child's brushing and rinsing (to minimize swallowing) |
| children under 2 years | ask a dentist or physician |
Inactive Ingredient
| Colloidal silicon Dioxide |
| Tribasic Calcium Phosphate |
| Chitosan |
| Xylitol |
| L-Menthol |
| steviol glycoside |
| Sodium Lauryl Sulfate |
| Dionized Water |
| Glycerine |
| D-sorbitol Solution |
| Sodium Carboxymethylcellulose |
| Alkyl(8-16) glcoside |
| Polyethylenglycol 4000 |
| Peppermint oil |
| Calendura extract |
| Licorice Extract |
| Moutan Root bark Extract |
| Grapefruit seed extract |
| Perilla herb extract |
| Propolis extract |
| SIRINMEDI ONE
sodium monofluorophosphate paste, dentifrice |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - Sungwon Pharmaceutical Co., Ltd. (689787898) |
| Registrant - Sungwon Pharmaceutical Co., Ltd. (689787898) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sungwon Pharmaceutical Co., Ltd. | 689787898 | manufacture(76058-100) | |
Revised: 3/2019
Document Id: 854041f9-55da-932c-e053-2a91aa0afb38
Set id: b0006c8c-8dea-48fc-8ed0-848574cc93a6
Version: 10
Effective Time: 20190329
Sungwon Pharmaceutical Co., Ltd.