Label: CHLORAMPHENICOL SODIUM SUCCINATE- chloramphenicol sodium succinate injection, powder, lyophilized, for solution
- NDC Code(s): 63323-011-15
- Packager: Fresenius Kabi USA, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 31, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
BOXED WARNING
(What is this?)
WARNING
Serious and fatal blood dyscrasias (aplastic anemia, hypoplastic anemia, thrombocytopenia and granulocytopenia) are known to occur after the administration of chloramphenicol. In addition, there have been reports of aplastic anemia attributed to chloramphenicol which later terminated in leukemia. Blood dyscrasias have occurred after both short-term and prolonged therapy with this drug. Chloramphenicol must not be used when less potentially dangerous agents will be effective, as described in the INDICATIONS AND USAGE section. It must not be used in the treatment of trivial infections or where it is not indicated, as in colds, influenza, infections of the throat; or as a prophylactic agent to prevent bacterial infections.
Precautions: It is essential that adequate blood studies be made during treatment with the drug. While blood studies may detect early peripheral blood changes, such as leukopenia, reticulocytopenia, or granulocytopenia, before they become irreversible, such studies cannot be relied on to detect bone marrow depression prior to development of aplastic anemia. To facilitate appropriate studies and observation during therapy, it is desirable that patients be hospitalized.
-
SPL UNCLASSIFIED SECTION
IMPORTANT CONSIDERATIONS IN PRESCRIBING INJECTABLE CHLORAMPHENICOL SODIUM SUCCINATE. CHLORAMPHENICOL SODIUM SUCCINATE IS INTENDED FOR INTRAVENOUS USE ONLY. IT HAS BEEN DEMONSTRATED TO BE INEFFECTIVE WHEN GIVEN INTRAMUSCULARLY.
- Chloramphenicol sodium succinate must be hydrolyzed to its microbiologically active form, and there is a lag in achieving adequate blood levels compared with the base given intravenously.
- Patients started on intravenous chloramphenicol sodium succinate should be changed to the oral form of another appropriate antibiotic as soon as practicable.
-
DESCRIPTION:
Chloramphenicol is an antibiotic that is clinically useful for, and should be reserved for, serious infections caused by organisms susceptible to its antimicrobial effects when less potentially hazardous therapeutic agents are ineffective or contraindicated. Sensitivity testing is essential to determine its indicated use, but may be performed concurrently with therapy initiated on clinical impression that one of the indicated conditions exists (see INDICATIONS AND USAGE section).
When reconstituted as directed, each vial contains a sterile solution equivalent to 100 mg of chloramphenicol per mL (1 g/10 mL).
Each gram (10 mL of a 10% solution) of chloramphenicol sodium succinate contains approximately 52 mg (2.25 mEq) of sodium.
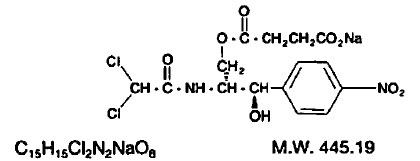
The chemical name for chloramphenicol sodium succinate is D-threo-(-)-2, 2-Dichloro-N-[β-hydroxy-α-(hydroxymethyl)-p-nitrophenethyl] acetamide α-(sodium succinate).
The structural formula is:
-
CLINICAL PHARMACOLOGY:
Chloramphenicol administered orally is absorbed rapidly from the intestinal tract. In controlled studies in adult volunteers using the recommended dosage of 50 mg/kg/day, a dosage of 1 g every 6 hours for 8 doses was given. Using the microbiological assay method, the average peak serum level was 11.2 mcg/mL one hour after the first dose. A cumulative effect gave a peak rise to 18.4 mcg/mL after the fifth dose of 1 g. Mean serum levels ranged from 8 to 14 mcg/mL over the 48-hour period. Total urinary excretion of chloramphenicol in these studies ranged from a low of 68% to a high of 99% over a three-day period. From 8% to 12% of the antibiotic excreted is in the form of free chloramphenicol; the remainder consists of microbiologically inactive metabolites, principally the conjugate with glucuronic acid. Since the glucuronide is excreted rapidly, most chloramphenicol detected in the blood is in the microbiologically active free form. Despite the small proportion of unchanged drug excreted in the urine, the concentration of free chloramphenicol is relatively high, amounting to several hundred mcg/mL in patients receiving divided doses of 50 mg/kg/day. Small amounts of active drug are found in bile and feces. Chloramphenicol diffuses rapidly, but its distribution is not uniform. Highest concentrations are found in liver and kidney, and lowest concentrations are found in brain and cerebrospinal fluid. Chloramphenicol enters cerebrospinal fluid even in the absence of meningeal inflammation, appearing in concentrations about half of those found in the blood. Measurable levels are also detected in pleural and in ascitic fluids, saliva, milk, and in the aqueous and vitreous humors. Transport across the placental barrier occurs with somewhat lower concentration in cord blood of neonates than in maternal blood.
Microbiology
Mechanism of Action
Chloramphenicol is a broad-spectrum antibiotic originally isolated from Streptomyces venezuelae. It inhibits bacterial protein synthesis by interfering with the transfer of activated amino acids from soluble RNA to ribosomes. In vitro, chloramphenicol exerts mainly a bacteriostatic effect on a wide range of gram-negative and gram-positive bacteria.
Antimicrobial Activity
Chloramphenicol has been shown to be active against most strains of the following microorganisms, both in vitro and in clinical infections as described in the INDICATIONS AND USAGE section.
Aerobic gram-negative microorganisms
Haemophilus influenzae
Salmonella species, including Salmonella typhi
Other microorganisms
Lymphogranuloma-psittacosis group
Rickettsia
Susceptibility Testing Methods
When available, the clinical microbiology laboratory should provide cumulative reports of in vitro susceptibility test results for antimicrobial drugs used in local hospitals and practice areas to the physician as periodic reports that describe the susceptibility profile of nosocomial and community-acquired pathogens. These reports should aid the physician in selecting an antibacterial drug for treatment.
Dilution Techniques
Quantitative methods are used to determine antimicrobial minimum inhibitory concentrations (MICs). These MICs provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MICs should be determined using a standardized (broth and/or agar). 1,3 The MIC values should be interpreted according to the criteria provided in Table 1.
Diffusion Techniques
Quantitative methods that require measurement of zone diameters can also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. The zone size should be determined using a standardized test method. 2,3 This procedure uses paper disks impregnated with 30 mcg chloramphenicol to test the susceptibility of bacteria to chloramphenicol. The disc diffusion breakpoints should be interpreted according to the criteria provided in Table 1.
Table 1. Susceptibility Test Interpretive Criteria for Chloramphenicol
Pathogen
Minimum Inhibitory Concentrations
(mcg/mL)
Zone Diameters
(mm)
S
I
R
S
I
R
Salmonella spp.
< 8
16
> 32
> 18
13 to 17
< 12
Haemophilus influenzae
< 2
4
> 8
≥ 29
26 to 28
< 25
A report of Susceptible (S) indicates that the antimicrobial drug is likely to inhibit growth of the pathogen if the antimicrobial drug reaches the concentration usually achievable at the site of infection. A report of Intermediate (I) indicates that the result should be considered equivocal, and, if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated or in situations where a high dosage of the drug can be used. This category also provides a buffer zone that prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of Resistant (R) indicates that the antimicrobial drug is not likely to inhibit growth of the pathogen if the antimicrobial drug reaches the concentration usually achievable at the infection site; other therapy should be selected.
Quality ControlStandardized susceptibility test procedures require the use of laboratory controls to monitor and ensure the accuracy and precision of supplies and reagents used in the assay, and the techniques of the individuals performing the test. 1,2,3 Standard chloramphenicol powder should provide the following range of MIC values noted in Table 2. For the disc diffusion technique using the 30 mcg disk, the criteria in Table 2 should be achieved.
Table 2. Quality Control Parameters for Chloramphenicol
QC Strain
Minimum Inhibitory Concentrations
(mcg/mL)
Zone Diameters
(mm)
Escherichia coli ATCC 25922
2 to 8
21 to 27
Haemophilus influenzae ATCC 49247
0.25 to 1
31 to 40
-
INDICATIONS AND USAGE:
In accord with the concepts in the Warning Box and this INDICATIONS AND USAGE section, chloramphenicol must be used only in those serious infections for which less potentially dangerous drugs are ineffective or contraindicated. However, chloramphenicol may be chosen to initiate antibiotic therapy on the clinical impression that one of the conditions below is believed to be present; in vitro sensitivity tests should be performed concurrently so that the drug may be discontinued as soon as possible if less potentially dangerous agents are indicated by such tests. The decision to continue use of chloramphenicol rather than another antibiotic when both are suggested by in vitro studies to be effective against a specific pathogen should be based upon severity of the infection, susceptibility of the pathogen to the various antimicrobial drugs, efficacy of the various drugs in the infection, and the important additional concepts contained in the Warning Box above.
• Acute infections caused by Salmonella typhi*
It is not recommended for the routine treatment of the typhoid carrier state.
• Serious infections caused by susceptible strains in accordance with the concepts expressed above:
a) Salmonella species
b) H. influenzae, specially meningeal infections
c) Rickettsia
d) Lymphogranuloma-psittacosis group
e) Various gram-negative bacteria causing bacteremia, meningitis or other serious gram-negative infections.
f) Other susceptible organisms which have been demonstrated to be resistant to all other appropriate antimicrobial agents.
• Cystic fibrosis regimens
*In treatment of typhoid fever some authorities recommend that chloramphenicol be administered at therapeutic levels for 8 to 10 days after the patient has become afebrile to lessen the possibility of relapse.
-
CONTRAINDICATIONS:
Chloramphenicol is contraindicated in individuals with a history of previous hypersensitivity and/or toxic reaction to it. It must not be used in the treatment of trivial infections or where it is not indicated, as in colds, influenza, infections of the throat; or as a prophylactic agent to prevent bacterial infections.
-
PRECAUTIONS:
General
Repeated courses of chloramphenicol treatment should be avoided if at all possible. Treatment should not be continued longer than required to produce a cure with little or no risk or relapse of the disease.
Excessive blood levels may result from administration of the recommended dose to patients with impaired liver or kidney function. The dosage should be adjusted accordingly, or preferably, the blood concentration should be determined at appropriate intervals.
The use of this antibiotic, as with other antibiotics, may result in an overgrowth of nonsusceptible organisms, including fungi. If infections caused by nonsusceptible organisms appear during therapy, appropriate measures should be taken.
Laboratory Tests
Baseline blood studies should be followed by periodic blood studies approximately every two days during therapy. The drug should be discontinued upon appearance of reticulocytopenia, leukopenia, thrombocytopenia, anemia or any other blood study findings attributable to chloramphenicol. However, it should be noted that such studies do not exclude the possible later appearance of the irreversible type of bone marrow depression.
Drug Interactions
Concurrent therapy with other drugs that may cause bone marrow depression should be avoided.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No studies have been conducted in animals or humans to evaluate the possibility of these effects with chloramphenicol.
Pregnancy
Pregnancy Category C –
Animal reproduction studies have not been conducted with chloramphenicol. There are no adequate and well-controlled studies to establish safety of this drug in pregnancy. It is not known whether chloramphenicol can cause fetal harm when administered to a pregnant woman. Orally administered chloramphenicol has been shown to cross the placental barrier. Because of potential toxic effects on the fetus (see ADVERSE REACTIONS,“Gray Syndrome”), chloramphenicol should be given to a pregnant woman only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
Chloramphenicol is excreted in human milk following oral administration of the drug. Because of the potential for serious adverse reactions in nursing infants from chloramphenicol, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother (see ADVERSE REACTIONS, “Gray Syndrome”).
Pediatric Use
Precaution should be used in therapy of premature and full-term neonates and infants to avoid “gray syndrome” toxicity. Due to immature metabolic processes in the neonate and infant, excessive blood levels may result from administration of the recommended dose. The dosage should be adjusted accordingly or, preferable, the blood concentration should be determined at appropriate intervals (see ADVERSE REACTIONS, "Gray Syndrome").
See DOSAGE AND ADMINISTRATION for dosing information in the pediatric population.
Geriatric Use
Clinical studies of chloramphenicol sodium succinate did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Each gram (10 mL of a 10% solution) of chloramphenicol sodium succinate contains approximately 52 mg (2.25 mEq) of sodium.
-
ADVERSE REACTIONS:
Blood Dyscrasias
The most serious adverse effect of chloramphenicol is bone marrow depression. Serious and fatal blood dyscrasias (aplastic anemia, hypoplastic anemia, thrombocytopenia, and granulocytopenia) are known to occur after the administration of chloramphenicol. An irreversible type of marrow depression leading to aplastic anemia with a high rate of mortality is characterized by the appearance weeks or months after therapy of bone marrow aplasia or hypoplasia. Peripherally, pancytopenia is most often observed, but in a small number of cases only one or two of the three major cell types (erythrocytes, leukocytes, platelets) may be depressed.
A reversible type of bone marrow depression, which is dose related, may occur. This type of marrow depression is characterized by vacuolization of the erythroid cells, reduction of reticulocytes and leukopenia, and responds promptly to the withdrawal of chloramphenicol.
An exact determination of the risk of serious and fatal blood dyscrasias is not possible because of lack of accurate information regarding 1) the size of the population at risk, 2) the total number of drug-associated dyscrasias, and 3) the total number of non-drug associated dyscrasias.
In a report to the California State Assembly by the California Medical Association and the State Department of Public Health in January 1967, the risk of fatal aplastic anemia was estimated at 1:24,200 to 1:40,500 based on two dosage levels.
There have been reports of aplastic anemia attributed to chloramphenicol which later terminated in leukemia.
Paroxysmal nocturnal hemoglobinuria has been reported.
Gastrointestinal Reactions
Nausea, vomiting, glossitis and stomatitis, diarrhea and enterocolitis may occur in low incidence.
Neurotoxic Reactions
Headache, mild depression, mental confusion, and delirium have been described in patients receiving chloramphenicol. Optic and peripheral neuritis have been reported, usually following long-term therapy. If this occurs, the drug should be promptly withdrawn.
Hypersensitivity Reactions
Fever, macular and vesicular rashes, angioedema, urticaria, and anaphylaxis may occur. Herxheimer’s reactions have occurred during therapy for typhoid fever.
"Gray Syndrome"
Toxic reactions including fatalities have occurred in the premature and neonate; the signs and symptoms associated with these reactions have been referred to as the “gray syndrome.” One case of gray syndrome has been reported in a neonate born to a mother having received chloramphenicol during labor. One case has been reported in a 3-month-old infant. The following summarizes the clinical and laboratory studies that have been made on these patients:
a) In most cases therapy with chloramphenicol had been instituted within the first 48 hours of life.
b) Symptoms first appeared after 3 to 4 days of continued treatment with high doses of chloramphenicol.
c) The symptoms appeared in the following order:
(1) abdominal distension with or without emesis;
(2) progressive pallid cyanosis;
(3) vasomotor collapse, frequently accompanied by irregular respiration;
(4) death within a few hours of onset of these symptoms.
d) The progression of symptoms from onset to exitus was accelerated with higher dose schedules.
e) Preliminary blood serum level studies revealed unusually high concentrations of chloramphenicol (over 90 mcg/mL after repeated doses).
f) Termination of therapy upon early evidence of the associated symptomatology frequently reversed the process with complete recovery.
-
DOSAGE AND ADMINISTRATION:
Chloramphenicol, like other potent drugs, should be prescribed at recommended doses known to have therapeutic activity. Administration of 50 mg/kg/day in divided doses will produce blood levels of the magnitude to which the majority of susceptible microorganisms will respond.
As soon as feasible an oral dosage form of another appropriate antibiotic should be substituted for intravenous chloramphenicol sodium succinate.
The following method of administration is recommended:
Intravenously as a 10% (100 mg/mL) solution to be injected over at least a one-minute interval. This is prepared by the addition of 10 mL of an aqueous diluent such as water for injection or 5% dextrose injection.
Adults
Adults should receive 50 mg/kg/day in divided doses at 6-hour intervals. In exceptional cases patients with infections due to moderately resistant organisms may require increased dosage up to 100 mg/kg/day to achieve blood levels inhibiting the pathogen, but these high doses should be decreased as soon as possible. Adults with impairment of hepatic or renal function or both may have reduced ability to metabolize and excrete the drug. In instances of impaired metabolic processes, dosages should be adjusted accordingly. (See discussion under Neonates.) Precise control of concentration of the drug in the blood should be carefully followed in patients with impaired metabolic processes by the available microtechniques.
Pediatric Patients
Dosage of 50 mg/kg/day divided into 4 doses at 6-hour intervals yields blood levels in the range effective against most susceptible organisms. Severe infections (e.g., bacteremia or meningitis), especially when adequate cerebrospinal fluid concentrations are desired, may require dosage up to 100 mg/kg/day; however, it is recommended that dosage be reduced to 50 mg/kg/day as soon as possible. Children with impaired liver or kidney function may retain excessive amounts of the drug.
Neonates
(See section titled ‘‘Gray Syndrome’’ under ADVERSE REACTIONS.)
A total of 25 mg/kg/day in 4 equal doses at 6-hour intervals usually produces and maintains concentrations in blood and tissues adequate to control most infections for which the drug is indicated. Increased dosage in these individuals, demanded by severe infections, should be given only to maintain the blood concentration within a therapeutically effective range. After the first two weeks of life, full-term neonates ordinarily may receive up to a total of 50 mg/kg/day equally divided into 4 doses at 6-hour intervals. These dosage recommendations are extremely important because blood concentration in all premature and full-term neonates under two weeks of age differs from that of other infant neonates. This difference is due to variations in the maturity of the metabolic functions of the liver and the kidneys.
When these functions are immature (or seriously impaired in adults), high concentrations of the drug are found which tend to increase with succeeding doses.
Pediatric Patients with Immature Metabolic Processes
In young infants and other pediatric patients in whom immature metabolic functions are suspected, a dose of 25 mg/kg/day will usually produce therapeutic concentrations of the drug in the blood. In this group particularly, the concentration of the drug in the blood should be carefully followed by microtechniques.
-
HOW SUPPLIED:
Chloramphenicol Sodium Succinate for Injection, USP is freeze-dried in the vial. When reconstituted as directed, each vial contains a sterile solution equivalent to 100 mg of chloramphenicol per mL (1 g/10 mL).
Product
No.
NDC
No.
1115
63323-011-15
Available in packages of 10 vials.
Preservative Free.
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
-
REFERENCES:
- Clinical and Laboratory Standards Institute (CLSI), Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard – Tenth Edition. CLSI document M07-A10 [2015], Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA.
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk Diffusion Susceptibility Tests; Approved Standard – Twelfth Edition. CLSI document M02-A12 [2015]. Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA.
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; Twenty-sixth Informational Supplement. CLSI document M100-S26 [2016]. Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA.
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL - PRINCIPAL DISPLAY - Chloramphenicol Sodium Succinate 1 gram Vial Label
Chloramphenicol Sodium Succinate for Injection, USP
1 gram per vial
For intravenous use only.
Preservative free.
Rx only

PACKAGE LABEL - PRINCIPAL DISPLAY - Chloramphenicol Sodium Succinate 1 gram Vial Tray LabelChloramphenicol Sodium Succinate for Injection, USP
1 gram per vial
For intravenous use only.
Preservative free.
10 x 1 g vials
Rx only

-
INGREDIENTS AND APPEARANCE
CHLORAMPHENICOL SODIUM SUCCINATE
chloramphenicol sodium succinate injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63323-011 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORAMPHENICOL SODIUM SUCCINATE (UNII: 872109HX6B) (CHLORAMPHENICOL - UNII:66974FR9Q1) CHLORAMPHENICOL 1 g in 10 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63323-011-15 10 in 1 TRAY 01/12/2001 1 10 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA062365 01/12/2001 Labeler - Fresenius Kabi USA, LLC (608775388) Establishment Name Address ID/FEI Business Operations Fresenius Kabi USA, LLC 023648251 manufacture(63323-011)



