MENSTRUAL PAIN RELIEF- acetaminophen, pamabrom and pyrilamine maleate tablet, film coated
DOLGENCORP, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Dollar General 44-369
Uses
-
temporarily relieves these symptoms associated with menstrual periods:
- cramps
- headaches
- bloating
- backaches
- water-weight gain
- irritability
- muscular aches
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
-
more than 8 caplets in 24 hours, which is the maximum daily amount
-
with other drugs containing acetaminophen
-
3 or more alcoholic drinks every day while using this product
Do not use
-
with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
Ask a doctor before use if you have
-
liver disease
-
glaucoma
-
trouble urinating due to an enlarged prostate gland
-
a breathing problem such as emphysema or chronic bronchitis
Ask a doctor or pharmacist before use if you are
-
taking the blood thinning drug warfarin
-
taking sedatives or tranquilizers
When using this product
-
drowsiness may occur
-
avoid alcoholic drinks
-
excitability may occur, especially in children
-
alcohol, sedatives and tranquilizers may increase drowsiness
-
be careful when driving a motor vehicle or operating machinery
Stop use and ask a doctor if
-
pain gets worse or lasts more than 10 days
-
fever gets worse or lasts more than 3 days
-
new symptoms occur
-
redness or swelling is present
These could be signs of a serious condition.
Keep out of reach of children.
Overdose warning: Taking more than the recommended dose (overdose) may cause liver damage. In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away. Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Directions
-
do not take more than the recommended dose
(see overdose warning) -
adults and children 12 years and older:
-
take 2 caplets with water every 6 hours as needed
-
do not exceed 8 caplets in a 24-hour period or as directed by a doctor
-
children under 12 years: ask a doctor
Other information
-
store at 25ºC (77ºF); excursions permitted between 15°-30°C (59°-86°F)
-
see end flap for expiration date and lot number
Inactive ingredients
croscarmellose sodium, crospovidone, hypromellose, magnesium stearate, microcrystalline cellulose, polydextrose, polyethylene glycol, povidone, pregelatinized starch, silica gel, stearic acid, titanium dioxide, triacetin
Principal Display Panel
DG™
health
Compare to active ingredients in Pamprin® Multi-Symptom*
Maximum Strength • Multi-Symptom
Menstrual Pain Relief
Acetaminophen
Pamabrom • Pyrilamine maleate
Pain Reliever/Diuretic/Antihistamine
•Relieves cramps, bloating,
irritability, headache & backache
•Aspirin free
•Caffeine free
32 Caplets
*This product is not manufactured or distributed by Chattem, Inc., owner of the registered trademark Pamprin® Multi-Symptom.
50844 REV1013G36927
DISTRIBUTED BY DOLGENCORP, LLC
100 MISSIONS RIDGE
GOODLETTSVILLE, TN 37072
100% Quality Guaranteed (888)309-9030
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
Dollar General 44-369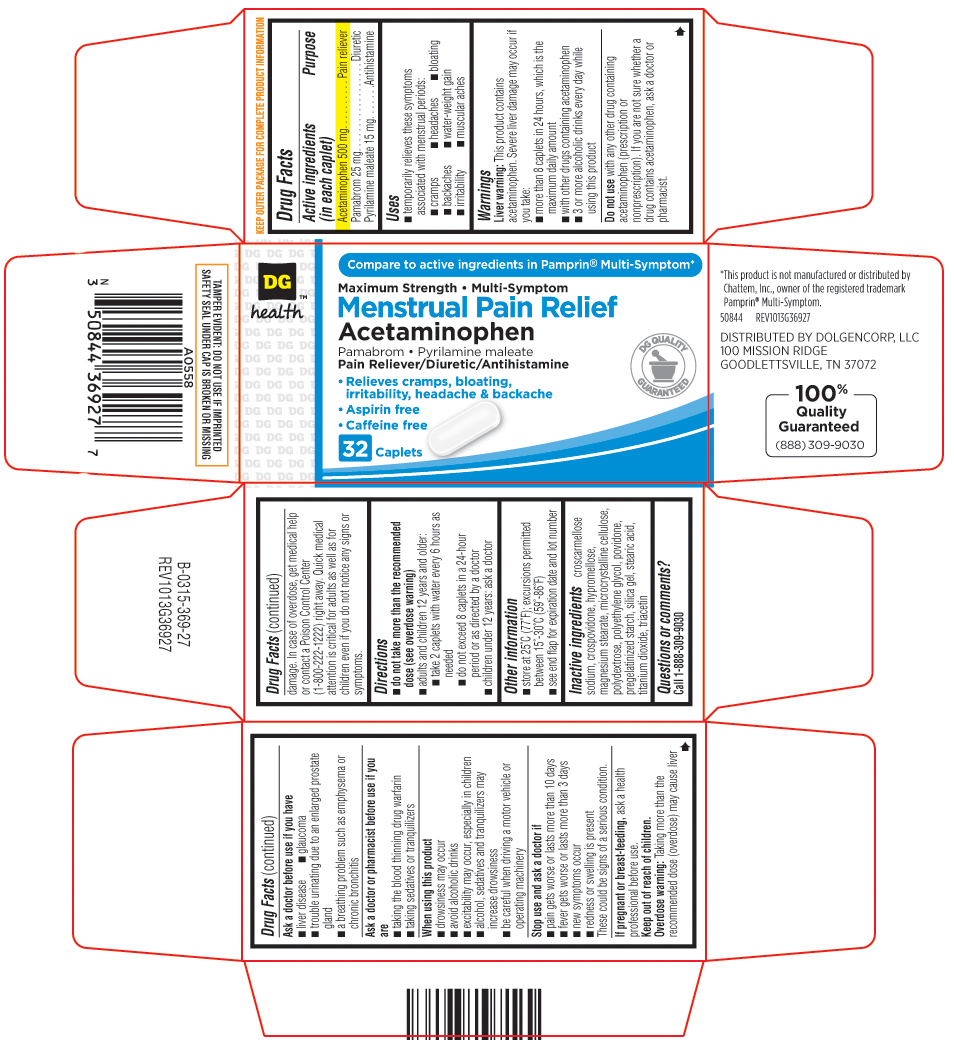
| MENSTRUAL PAIN RELIEF
acetaminophen, pamabrom and pyrilamine maleate tablet, film coated |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - DOLGENCORP, LLC (068331990) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867894 | MANUFACTURE(55910-369) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 038154464 | PACK(55910-369) | |