SHEERDESENZ DESENSITIZING TREATMENT- potassium nitrate film
CAO Group, Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Product Information
[Patient Kit Information Panel]
TO REORDER: 1.877.236.4408
www.caogroup.com
PACKAGE CONTENTS: 4) desensitizing treatments
See package insert for dosage information. Rx Only. Dispose of properly after use. For complete safety information see product MSDS. Do not use if the packaging has been damaged, or if the safety seals are found to be broken.
REORDER #: 006-00104
Manufactured by CAO (China) Medical Equipment Co., Ltd. for CAO Group, Inc.
4628 West Skyhawk Drive
West Jordan, UT 84084
U.S.A.
877-236-4408 (tel)
801-256-9287 (fax)
www.caogroup.com
Expiration date:
MADE IN CHINA
[Side panels for the Patient Pack]
SheerDesenZ
Active Ingredient: Potassium nitrate, 12mg/film
Other Ingredients: Proprietary polymer, water/eau, xylitol, glycerin, poly(acrylic acid), peppermint oil, sucralose, potassium hydroxide
NDC#: 1406000304
Description
Sheer DesenZ™ is the easiest way to provide relief from tooth pain and sensitivity. Just place on the tooth and dismiss the patient. The large film provides quadrant coverage. The small films are for specific teeth with sensitivity or, when combined with the large film, to provide full arch coverage. These films are meant to be worn up to 60 minutes and then removed.
Counter Indications
• Do not smoke while wearing the film.
• Avoid eating while wearing the film.
• Flush with copious amounts of water if accidentally placed in eye.
• Keep out of reach of young children.
• Avoid contact with clothing.
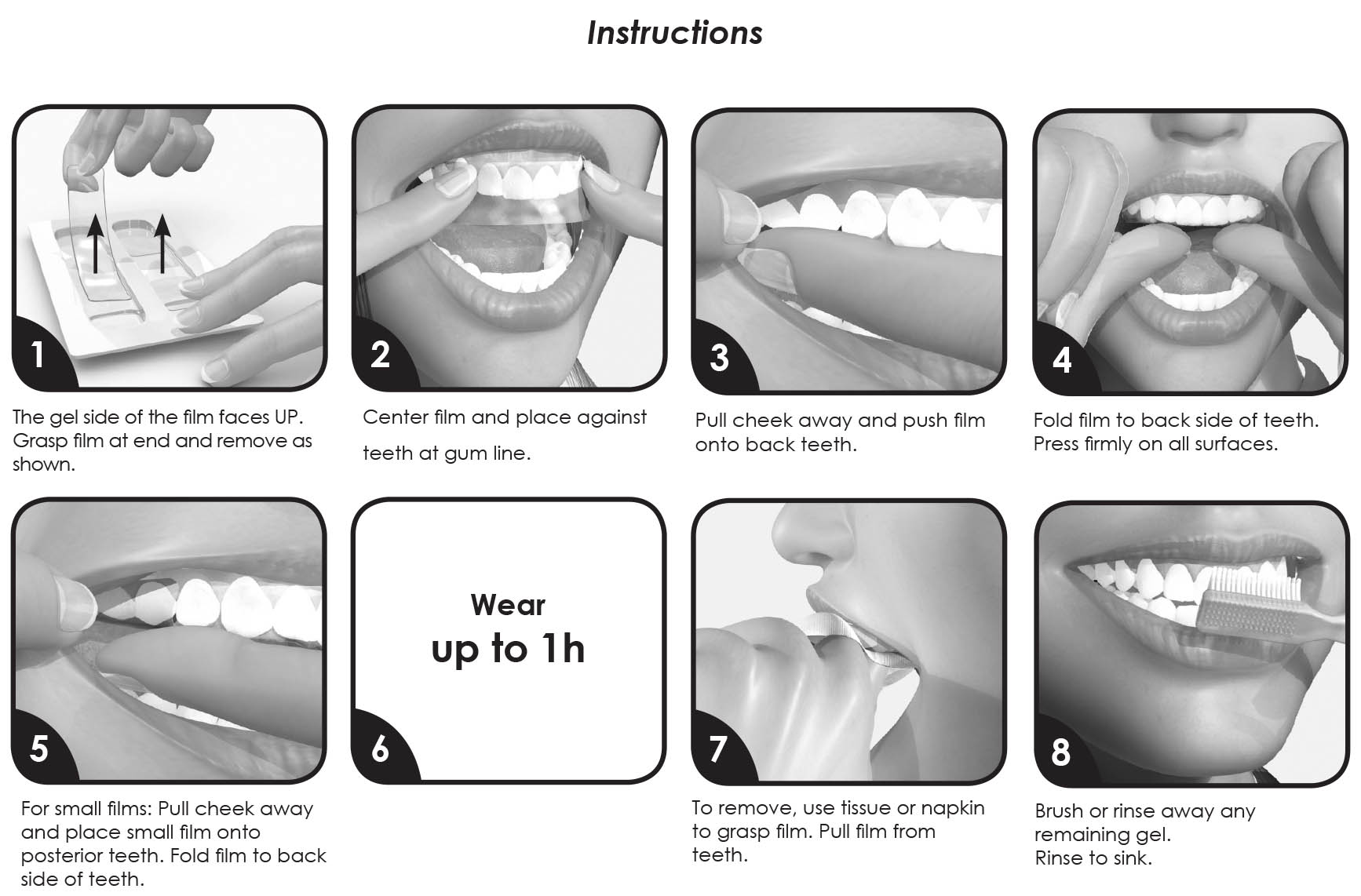
Instructions
- The gel side of the film faces UP. Grasp film at end and remove as shown.
- Center film and place against teeth at gum line.
- Pull cheek away and push film onto back teeth.
- Fold film to back side of teeth. Press firmly on all surfaces.
- For small films: Pull cheek away and place small film onto posterior teeth. Fold film to back side of teeth.
- Wear up to 1 hour.
- To remove, use tissue or napkin to grasp film. Pull film from teeth.
- Brush or rinse away any remaining gel. Rinse to sink.
Hyperkalemia warning:
Use of this product may cause hyperkalemia, a serious condition that must be treated promptly because it can affect cell function and proper heart function. This can occur even if you have used this product before. Stop use and seek immediate medical attention if the patient develops:
- muscle fatigue
- weakness
- abnormal heart beat (arrhythnmias)
- paralysis
- nausea
[Complete Instructions Back Page]
Caution: Federal law restricts this device to sale by or on the order of a licensed professional.
Disclaimer: CAO Group believes this information to be accurate and is offered only for the benefit of its customers for use of the product under proscribed conditions. This document is not to be considered a warranty or guarantee of product performance, and CAO Group is not legally bound to such claims based on this document.
Manufactured by CAO (China) Medical Equipment Co,. Ltd. for
CAO Group, Inc.
P 877.236.4408 F 801.256.9287
4628 West Skyhawk Drive, West Jordan, UT 84084-4501 U.S.A.
www.caogroup.com
13 Sept 2010
SAL-CAI010 REV 06.18.01 20JUN2018
| SHEERDESENZ
DESENSITIZING TREATMENT
potassium nitrate film |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - CAO Group, Inc. (102422578) |

