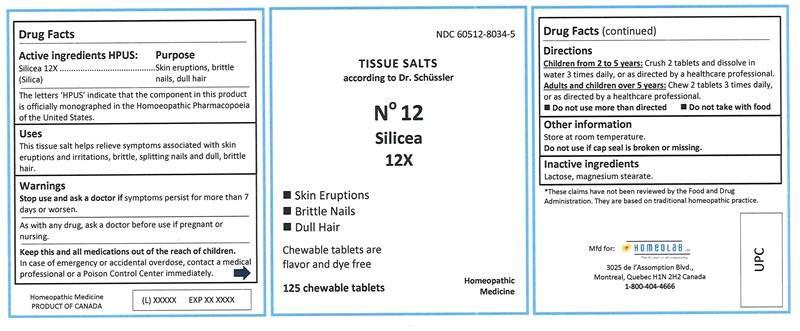
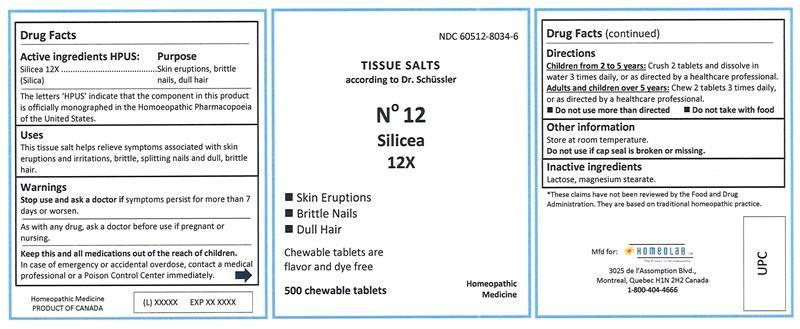
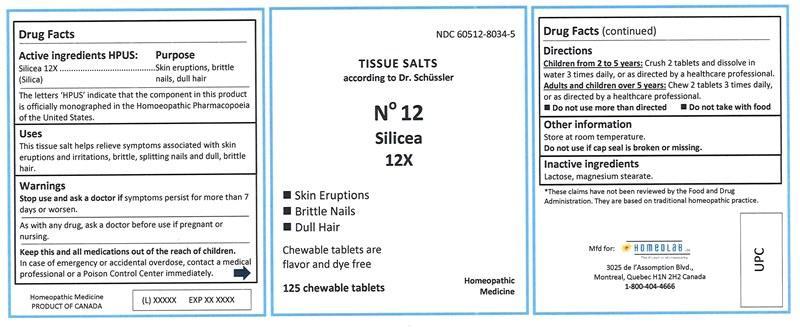
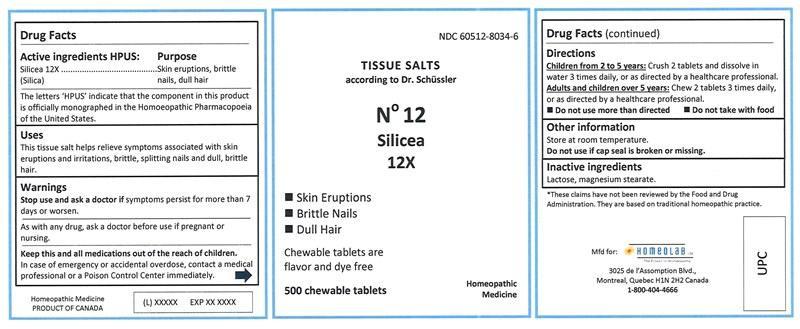
Label: SILICEA tablet, chewable
-
Contains inactivated NDC Code(s)
NDC Code(s): 60512-8034-5, 60512-8034-6 - Packager: HOMEOLAB USA INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated March 26, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients HPUS
- Purpose
- REFERENCES
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- LABEL
-
INGREDIENTS AND APPEARANCE
SILICEA
silicea tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:60512-8034 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (COLLOIDAL SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 12 [hp_X] Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white Score no score Shape ROUND Size 8mm Flavor Imprint Code NONE Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:60512-8034-5 125 in 1 JAR 2 NDC:60512-8034-6 500 in 1 JAR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 03/26/2014 Labeler - HOMEOLAB USA INC (202032533) Establishment Name Address ID/FEI Business Operations HOMEOLAB USA INC 202032533 manufacture(60512-8034)