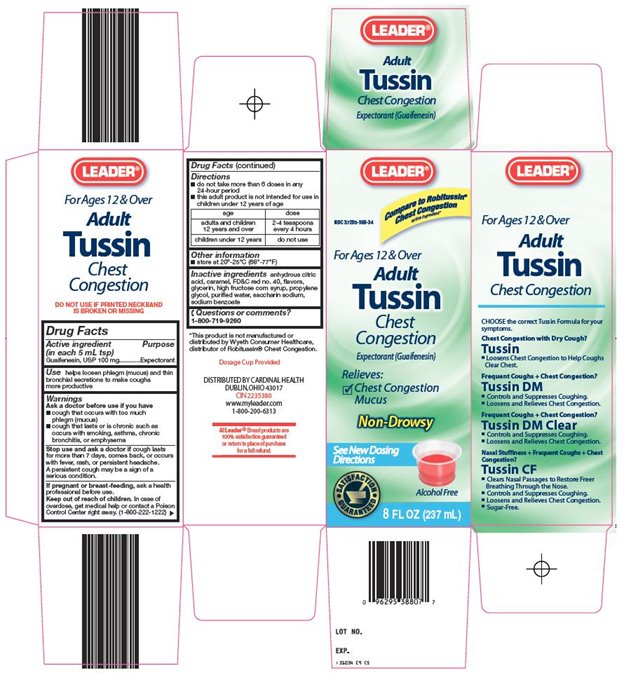
LEADER ADULT TUSSIN CHEST CONGESTION- guaifenesin syrup
Cardinal Health
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Cardinal Health Tussin Drug Facts
Warnings
Ask a doctor before use if you have
- •
- cough that occurs with too much phlegm (mucus)
- •
- cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis, or emphysema
Directions
- •
- do not take more than 6 doses in any 24-hour period
- •
- this adult product is not intended for use in children under 12 years of age
|
age |
dose |
|
adults and children 12 years and over |
2-4 teaspoons every 4 hours |
|
children under 12 years |
do not use |
| LEADER ADULT TUSSIN
CHEST CONGESTION
guaifenesin syrup |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Cardinal Health (097537435) |
Revised: 11/2017
Document Id: de409e0d-b1ff-49c0-9427-3581c326523d
Set id: ac6f7da8-879b-47da-bb95-229cc828d16c
Version: 2
Effective Time: 20171113
Cardinal Health