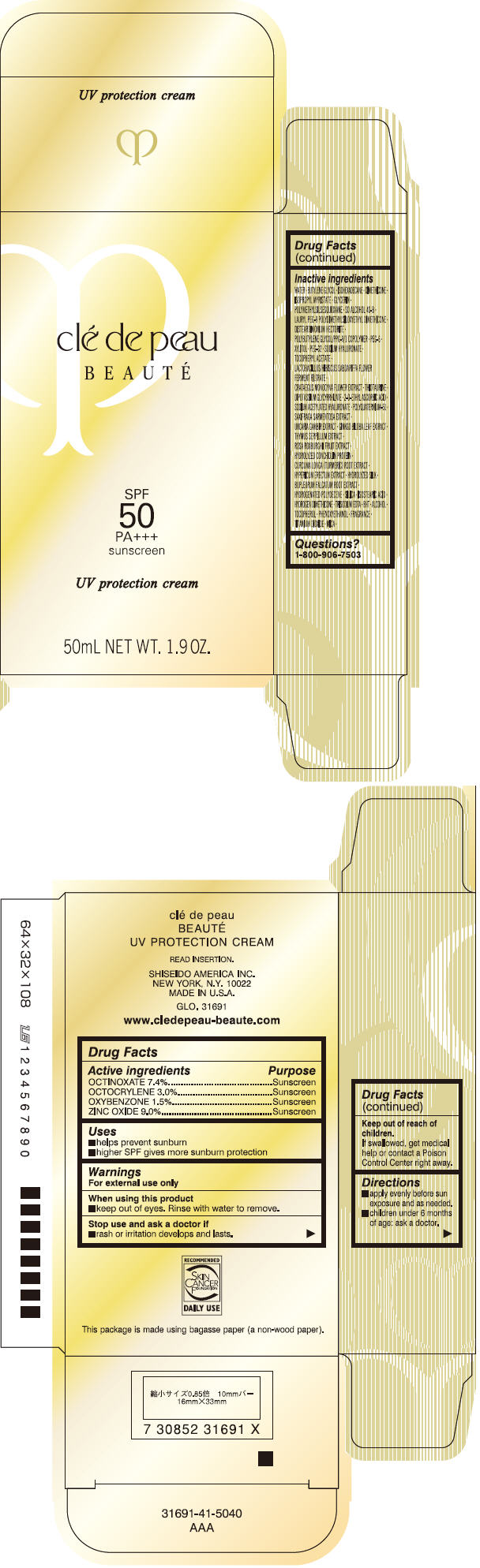
CLE DE PEAU BEAUTE UV PROTECTION- octinoxate, octocrylene, oxybenzone, and zinc oxide cream
SHISEIDO AMERICA INC.
----------
| Active ingredients | Purpose |
| OCTINOXATE 7.4% | Sunscreen |
| OCTOCRYLENE 3.0% | Sunscreen |
| OXYBENZONE 1.5% | Sunscreen |
| ZINC OXIDE 9.0% | Sunscreen |
Uses
- helps prevent sunburn
- higher SPF gives more sunburn protection
Warnings
For external use only
When using this product
- keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if
- rash or irritation develops and lasts.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- apply evenly before sun exposure and as needed.
- children under 6 months of age: ask a doctor.
Inactive Ingredients
WATER • BUTYLENE GLYCOL • ISOHEXADECANE • DIMETHICONE • ISOPROPYL MYRISTATE • GLYCERIN • POLYMETHYLSILSESQUIOXANE • SD ALCOHOL 40-B • LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE • DISTEARDIMONIUM HECTORITE • POLYBUTYLENE GLYCOL/PPG-9/1 COPOLYMER • PEG-6 • XYLITOL • PEG-32 • SODIUM HYALURONATE • TOCOPHERYL ACETATE • LACTOBACILLUS/HIBISCUS SABDARIFFA FLOWER FERMENT FILTRATE • CRATAEGUS MONOGYNA FLOWER EXTRACT • THIOTAURINE • DIPOTASSIUM GLYCYRRHIZATE • 2-O-ETHYL ASCORBIC ACID • SODIUM ACETYLATED HYALURONATE • POLYQUATERNIUM-51 • SAXIFRAGA SARMENTOSA EXTRACT • UNCARIA GAMBIR EXTRACT • GINKGO BILOBA LEAF EXTRACT • THYMUS SERPILLUM EXTRACT • ROSA ROXBURGHII FRUIT EXTRACT • HYDROLYZED CONCHIOLIN PROTEIN • CURCUMA LONGA (TURMERIC) ROOT EXTRACT • HYPERICUM ERECTUM EXTRACT • HYDROLYZED SILK • BUPLEURUM FALCATUM ROOT EXTRACT • HYDROGENATED POLYDECENE • SILICA • ISOSTEARIC ACID • HYDROGEN DIMETHICONE • TRISODIUM EDTA • BHT • ALCOHOL • TOCOPHEROL • PHENOXYETHANOL • FRAGRANCE • TITANIUM DIOXIDE • MICA •
Questions?
1-800-906-7503
PRINCIPAL DISPLAY PANEL - 50 mL Carton
clé de peau
BEAUTÉ
SPF
50
PA+++
sunscreen
UV protection cream
50mL NET WT. 1.9 OZ.
SHISEIDO AMERICA INC.