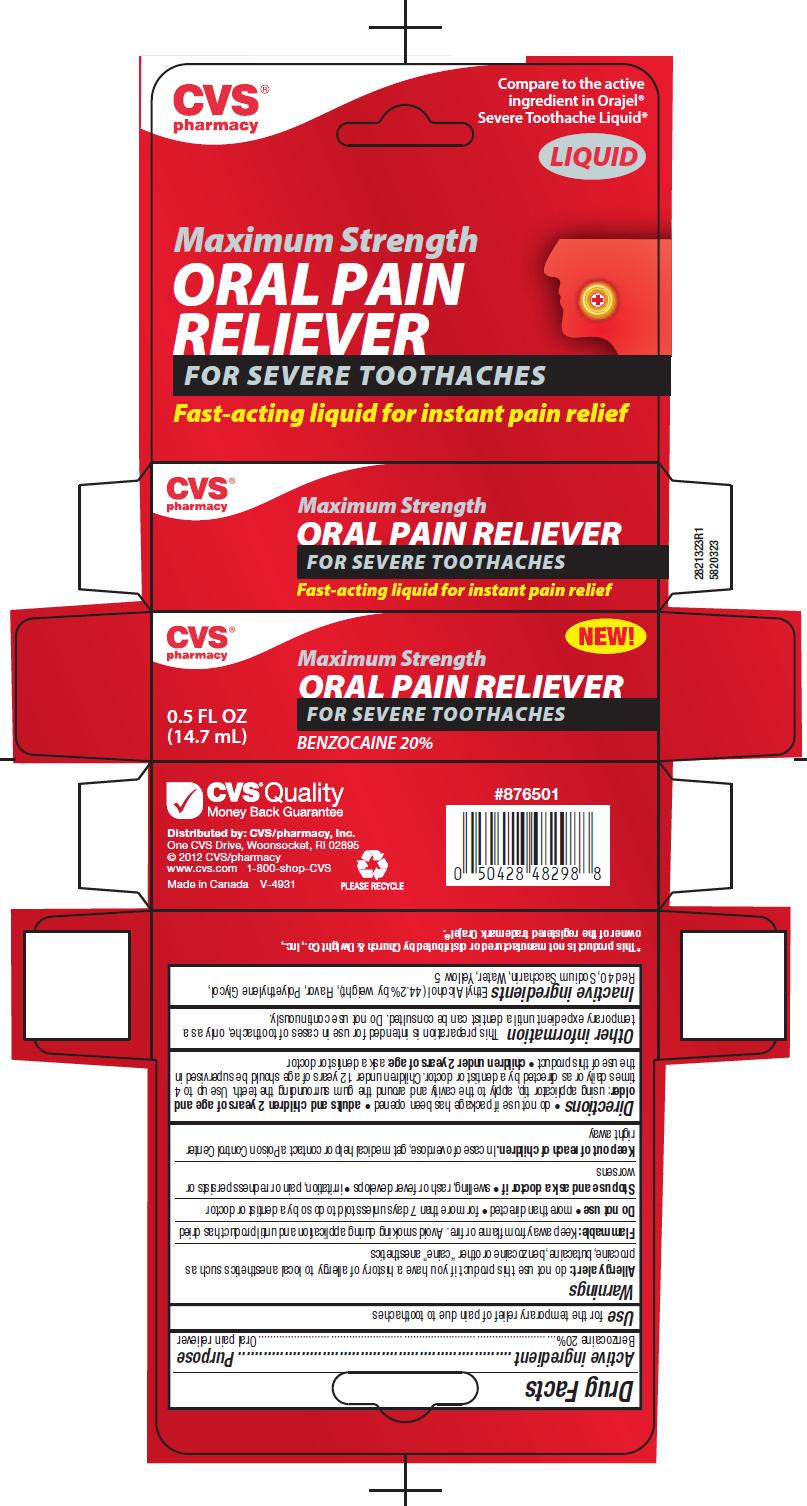
SEVERE ORAL PAIN RELIEVER- benzocaine liquid
CVS Pharmacy
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
CVS Max Str Oral Pain Reliever Severe
Warnings
Allergy alert: do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or other “caine” anesthetics
Flammable: Keep away from flame or fire. Avoid smoking during application and until product has dried
Directions
- do not use if package has been opened
- adults and children 2 years of age and older: using applicator tip, apply to the cavity and around the gum surrounding the teeth. Use up to 4 times daily or as directed by a dentist or doctor. Children 12 years of age should be supervised in the use of this product.
- children under 2 years of age: ask a dentist or doctor
Other Information
This preparation is intended for use in cases of toothache, only as a temporary expedient until a dentist can be consulted. Do not use continiously.
| SEVERE ORAL PAIN RELIEVER
benzocaine liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - CVS Pharmacy (062312574) |
| Registrant - Lornamead (126440440) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| CSR Cosmetic Solutions | 243501959 | manufacture(59779-820) | |
Revised: 2/2019
Document Id: 81e0c804-de70-0c48-e053-2991aa0a0f72
Set id: a87ae6a3-0c00-4d2c-9663-50cb600526a2
Version: 6
Effective Time: 20190214
CVS Pharmacy