STOOL SOFTENER- docusate sodium capsule, liquid filled
EQUATE (Walmart Stores, Inc.)
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Stool Softener
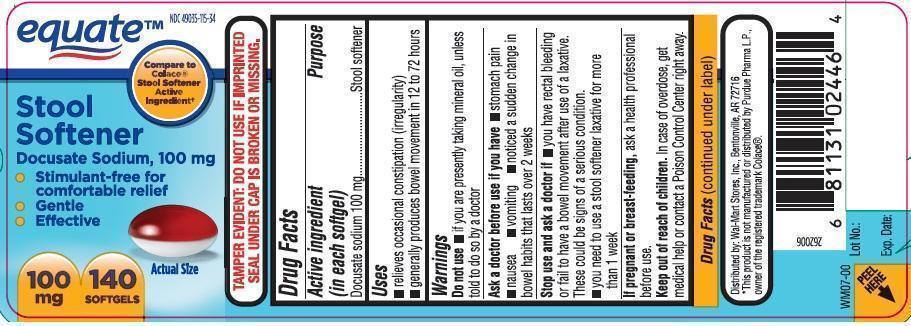
Uses
- relieves occasional constipation (irregularity)
- generally produces a bowel movement in 12 to 72 hours
Warnings
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel movements that lasts over 2 weeks
Stop use and ask a doctor if
- you have rectal bleeding or fail to have a bowel movement after using a laxative. These could be signs of a serious condition.
- you need to use a stool softener laxative for more than 1 week
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Inactive ingredients
D&C Red #33, FD&C blue #1, FD&C Red #40, FD&C Yellow #6, gelatin, glycerin, polyethylene glycol, propylene glycol, sorbitol special, citric acid, purified water and white edible ink
Principal Display Panel
Stool Softener
Docusate Sodium, 100 mg
Compare to Colacel® Stool Softener Active Ingredient*
60 Softgels
NDC 49035-115-22
Distributed by: Wal-Mart Stores, Inc.
Bentonville, AR 72716
*This product is not manufactured or distributed by Purdue Pharma L.P, owner of the registered trademark Colace®.

Principal Display Panel
Stool Softener
Docusate Sodium, 100 mg
Compare to Colacel® Stool Softener Active Ingredient*
140 Softgels
NDC 49035-115-34
Distributed by: Wal-Mart Stores, Inc.
Bentonville, AR 72716
*This product is not manufactured or distributed by Purdue Pharma L.P, owner of the registered trademark Colace®.

Principal Display Panel
Stool Softener
Docusate Sodium, 100 mg
Compare to Colacel® Stool Softener Active Ingredient*
280 Softgels
NDC 49035-115-35
Distributed by: Wal-Mart Stores, Inc.
Bentonville, AR 72716
*This product is not manufactured or distributed by Purdue Pharma L.P, owner of the registered trademark Colace®.

| STOOL SOFTENER
docusate sodium capsule, liquid filled |
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| Labeler - EQUATE (Walmart Stores, Inc.) (051957769) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Humanwell PuraCap Pharmaceutical (Wuhan) Co., Ltd | 421293287 | analysis(49035-115) , manufacture(49035-115) | |