APAP- acetaminophen tablet
Contract Pharmacal Corp.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
1190
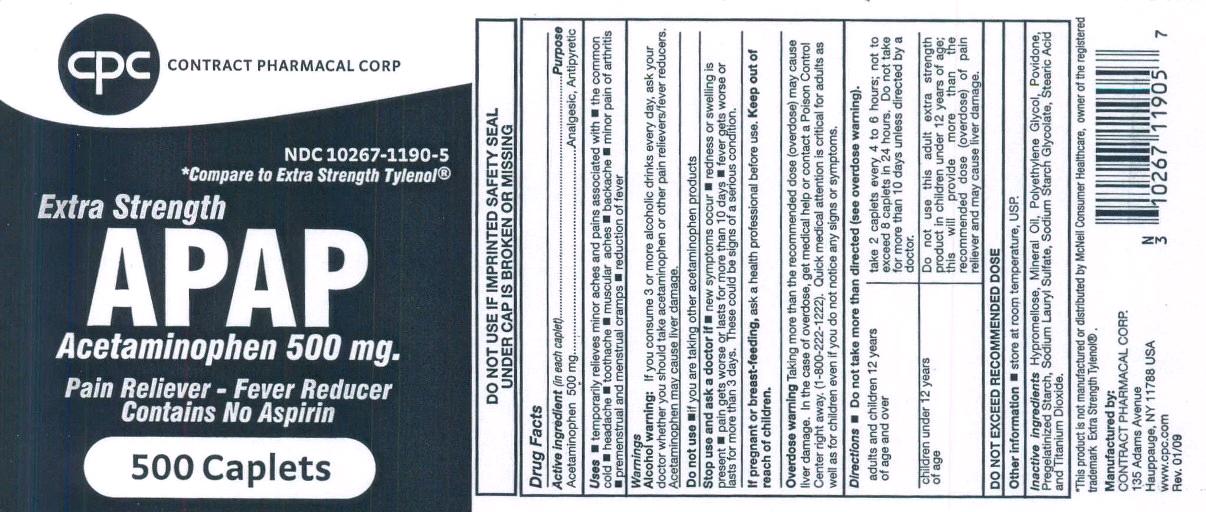
Uses
- temporarily relieves minor aches and pains associated with
- the common cold
- headache
- toothache
- muscular aches
- backache
- minor pain of arthritis
- premenstrual and menstrual cramps
- reduction of fever
Warnings
Alcohol warning: If you consume 3 or more alcoholic drinks every day, ask your doctor whether you should take acetaminophen or other pain relievers/fever reducers.
Acetaminophen may cause liver damage.
Stop use and ask a doctor if
- new symptoms occur
- redness or swelling is present
- pain gets worse or lasts for more than 10 days
- fever gets worse or lasts more than 3 days
These could be signs of a serious condition.
Keep out of reach of children.
Overdose warning Taking more than the recommended dose (overdose) may cause liver damage. In case of In case of overdose, get medical help or contact Poison Control Center right away. (1-800-222-1222) Quick medical attention is critical for adults as well as children even if you do not notice any signs or symptoms.
Directions
| adults and children 12 years of age and over | take 2 tablets every 4 hour to 6 hours, not to exceed 8 tablets in 24 hours. Do not take for more than 10 days unless directed by a doctor. |
| children under 12 years of age | Do not use Extra Strength product in children 12 years of age; this will provide more than the recommended dose (overdose) of pain reliever and may cause liver damage. |
DO NOT EXCEED RECOMMENDED DOSE
Other information
- store at room temperature, USP
Inactive Ingredients
Hypromellose, Mineral Oil, Polyethylene Glycol, Povidone, Pregelatinized Starch, Sodium Lauryl Sulfate, Sodium Starch Glycolate, Stearic Acid and Titanium Dioxide.
*This product is not manufactured or distributed by McNeil Consumer Healthcare, owner of the registered trademark Extra Strength Tylenol®.
Manufactured by:
CONTRACT PHARMACAL CORP.
135 Adams Avenue
Hauppauge, NY 11788 USA
www.CPCHealth.com
Rev. 01/09
| APAP
acetaminophen tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Contract Pharmacal Corp. (057795122) |
| Registrant - Contract Pharmacal Corp. (057795122) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Contract Pharmacal Corp | 968334974 | manufacture(10267-1190) | |