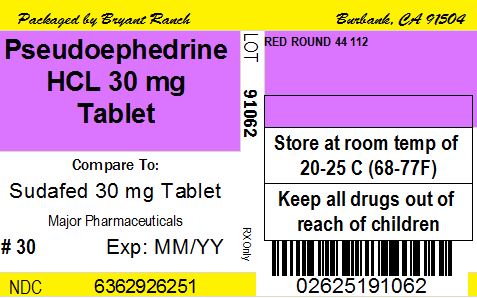
NASAL DECONGESTANT - pseudoephedrine hcl tablet, sugar coated
Bryant Ranch Prepack
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
DRUG FACTS
- temporarily relieves nasal congestion due to the common cold, hay fever, or other upper respiratory allergies
- temporarily restores freer breathing through the nose
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- high blood pressure
- heart disease
- thyroid disease
- diabetes
- trouble urinating due to an enlarged prostate gland
- you get nervous, dizzy or sleepless
- symptoms do not improve within 7 days or accompanied by fever
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directionstake every 4-6 hoursdo not take more than 4 doses in 24 hoursadults and children 12 years and over: 2 tabletschildren 6 to under 12 years: 1 tabletchildren under 6 years: do not use
- store at 25°C (77°F), excursions permitted between 15°-30°C (59°-86°F)
- protect from light
Inactive ingredients acacia, calcium sulfate anhydrous, carnauba wax, colloidal silicon dioxide, corn starch, D-C red #27 aluminum lake, D-C yellow #10 aluminum lake, FD-C red #40 aluminum lake, FD-C yellow #6 aluminum lake, iron oxide black, iron oxide red, iron oxide yellow (iron oxide ochre), kaolin, microcrystalline cellulose, polyethylene glycol (PEG) - 400, pregelatinized starch, shellac, sodium starch glycolate, stearic acid, sugar, talc, titanium dioxide
| NASAL DECONGESTANT
pseudoephedrine hcl tablet, sugar coated |
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Bryant Ranch Prepack (171714327) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bryant Ranch Prepack | 171714327 | REPACK(63629-2625) , RELABEL(63629-2625) | |