PAIN RELIEF- menthol, methyl salicylate cream
CVS Pharmacy
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
CVS Pharmacy, Inc. Pain Relief Drug Facts
Uses
temporarily relieves minor pain associated with
- •
- arthritis
- •
- simple backache
- •
- strains
- •
- sprains
- •
- bruises
Warnings
For external use only
When using this product
- •
- use only as directed
- •
- do not bandage tightly
- •
- do not use with a heating pad
- •
- avoid contact with the eyes or mucous membranes
- •
- do not apply to wounds or damaged or irritated skin
Directions
adults and children over 12 years
- •
- apply generously to affected area
- •
- massage into painful area until thoroughly absorbed into skin
- •
- repeat as necessary, not more than 3 to 4 times daily
children 12 years or younger ask a doctor
Inactive ingredients
carbomer homopolymer type C, cetyl esters wax, emulsifying wax, oleth-3 phosphate, purified water, stearic acid, trolamine
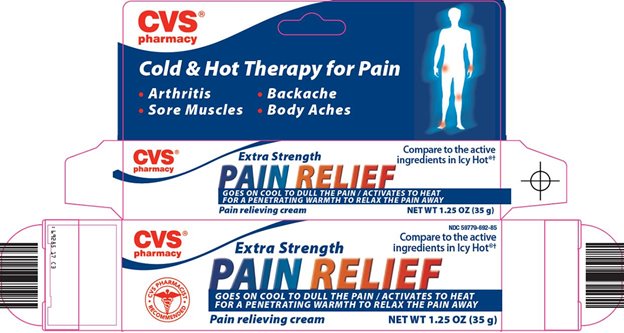
Principal Display Panel
Cold & Hot Therapy for Pain
Arthritis
Backache
Sore Muscles
Body Aches
Extra Strength
Compare to the active ingredients in Icy Hot®
PAIN RELIEF
GOES ON COOL TO DULL THE PAIN / ACTIVATES TO HEAT FOR A PENETRATING WARMTH TO RELAX THE PAIN AWAY
Pain relieving cream
Pain Relief Carton Image 2
| PAIN RELIEF
menthol, methyl salicylate cream |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - CVS Pharmacy (062312574) |