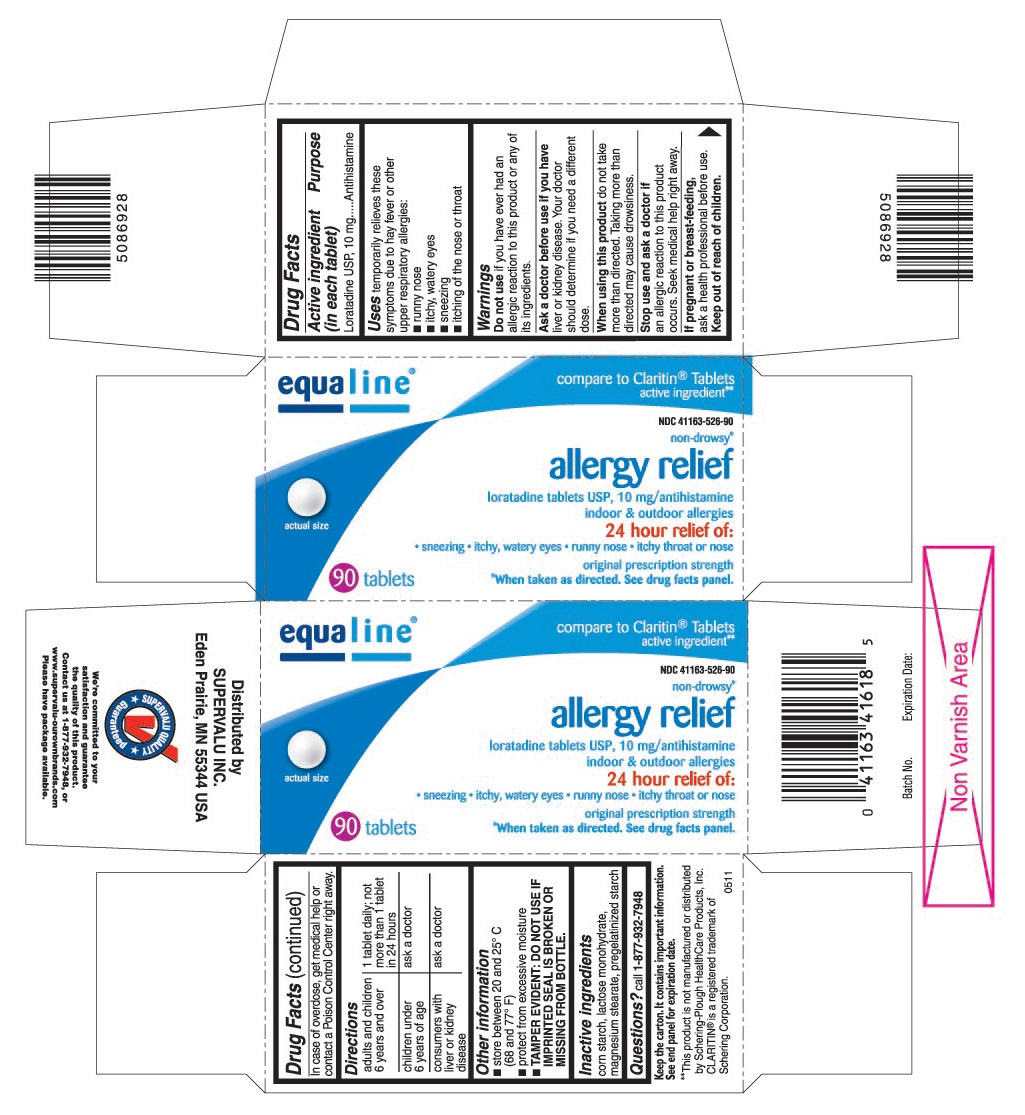
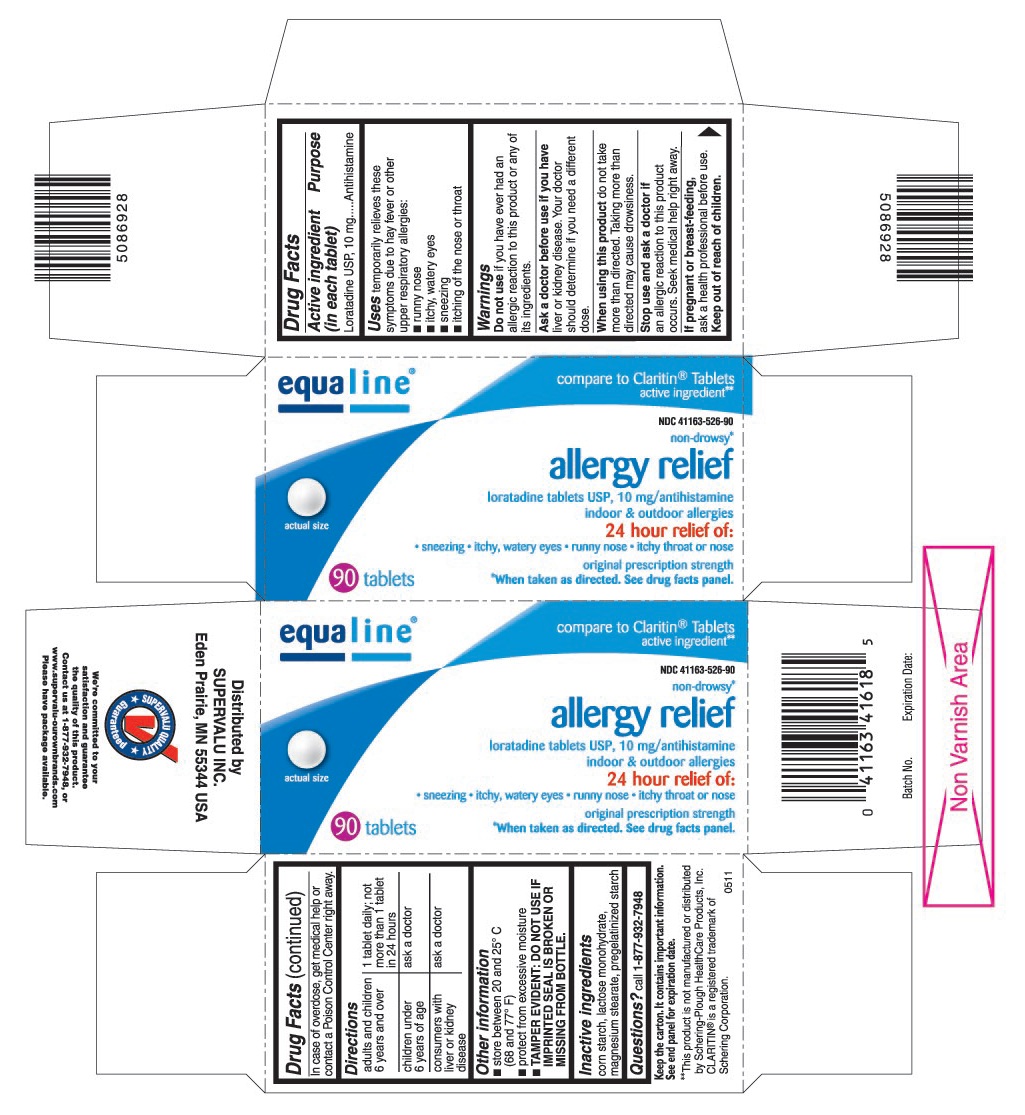
Label: LORATADINE tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 41163-526-21, 41163-526-30, 41163-526-60, 41163-526-69, view more41163-526-90 - Packager: SUPERVALU INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 6, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT (IN EACH TABLET)
- PURPOSE
- USES
-
WARNINGS
Ask a doctor before use if you have
liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
do not take more than directed. Taking more than directed may cause drowsiness.
- DIRECTIONS
- OTHER INFORMATION
- INACTIVE INGREDIENTS
- QUESTIONS?
-
PRINCIPAL DISPLAY PANEL
loratadine tablets USP, 10 mg/antihistamine
- sneezing
- itchy, watery eyes
- runny nose
- itchy throat or nose
original prescription strength
*When taken as directed. See drug facts panel.
compare to Claritin®Tablets active ingredient**
**This product is not manufactured or distributed by Schering-Plough HealthCare Products, Inc. CLARITIN®is a registered trademark of Schering Corporation.
-
INGREDIENTS AND APPEARANCE
LORATADINE
loratadine tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41163-526 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LORATADINE (UNII: 7AJO3BO7QN) (LORATADINE - UNII:7AJO3BO7QN) LORATADINE 10 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) STARCH, PREGELATINIZED CORN (UNII: O8232NY3SJ) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white (White to Off-White) Score no score Shape ROUND Size 6mm Flavor Imprint Code RX526 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41163-526-69 10 in 1 BLISTER PACK 2 NDC:41163-526-21 20 in 1 BLISTER PACK 3 NDC:41163-526-30 30 in 1 BOTTLE 4 NDC:41163-526-60 60 in 1 BOTTLE 5 NDC:41163-526-90 90 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076134 08/19/2003 Labeler - SUPERVALU INC. (006961411) Registrant - Ranbaxy Pharmaceuticals Inc. (937890044) Establishment Name Address ID/FEI Business Operations Ohm Laboratories Inc. 051565745 manufacture(41163-526)