STOOL SOFTENER- docusate sodium and sennosides tablet, coated
PLD Acquisitions LLC DBA Avéma Pharma Solutions
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
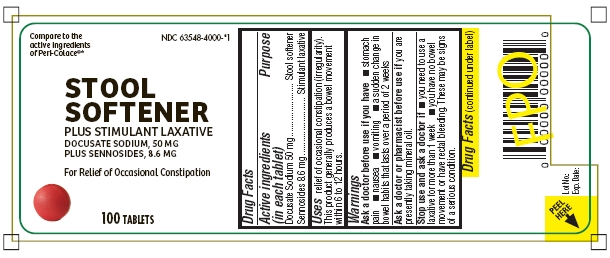
Active ingredients (in each tablet)
Docusate Sodium 50 mg*
Sennosides 8.6 mg**
Purpose
*Stool softener
**Stimulant laxative
Uses
relief of occasional constipation (irregularity). This product generally produces a bowel movement within 6 to 12 hours.
Warnings
Ask a doctor before use if you have
- stomach
- pain
- nausea
- vomiting
- a sudden change in bowel habits that lasts over a period of 2 weeks
ask a doctor or pharmacist before use if
Ask a doctor or pharmacist before use if you are presently taking mineral oil.
Stop use and ask a doctor if
- you need to use a laxative for more than 1 week
- you have no bowel movement or have rectal bleeding. These may be signs of a serious condition.
if pregnant or breast-feeding
If pregnant or breast-feeding, ask a health care professional before use.
keep out of reach of children
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- doses may be taken as a single dose or in divided doses preferably in the evening
| adults and children 12 years of age and older | take 2-4 tablets daily |
| children 6 to under 12 years of age | take 1-2 tablets daily |
| children 2 to 6 years of age | take up to 1 tablet daily |
| children under 2 years of age | ask a doctor |
Other information
- each tablet contains: sodium 6 mg/tablet VERY LOW SODIUM
- each tablet contains: calcium 20 mg/tablet
- store at 15°-30°C (59°-86°F), protect from excessive moisture
- do not use if imprinted safety seal under cap is broken or missing
- *This product is not manufactured or distributed by Purdue Pharma L.P., owner of the registered trademark Peri-Colace®.
Inactive ingredients
carnauba wax, colloidal silicon dioxide, croscarmellose sodium, dibasic calcium phosphate dihydrate, FD&C Red #40 Aluminum Lake, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol (PEG) 400, sodium benzoate, stearic acid, and titanium dioxide. May also contain: ethanol, FD&C Blue # 2 Aluminum Lake, maltodextrin, mineral oil, Opadry ll Red, pregelatinized starch, silicon dioxide 50s.
Package/Label Principal Display Panel
NDC 63548-4000-*1
Compare to the active ingredients of Peri-Colace®*
STOOL SOFTENER PLUS STIMULANT LAXATIVE
DOCUSATE SODIUM, 50 MG
PLUS SENNOSIDES, 8.6 MG
For Relief of Occasional Constipation
100 TABLETS