Label: BODYCOLOGY TOASTED VANILLA SUGAR KIT- toasted vanilla sugar kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 51345-065-01, 51345-066-01 - Packager: Advanced Beauty Systems, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 5, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
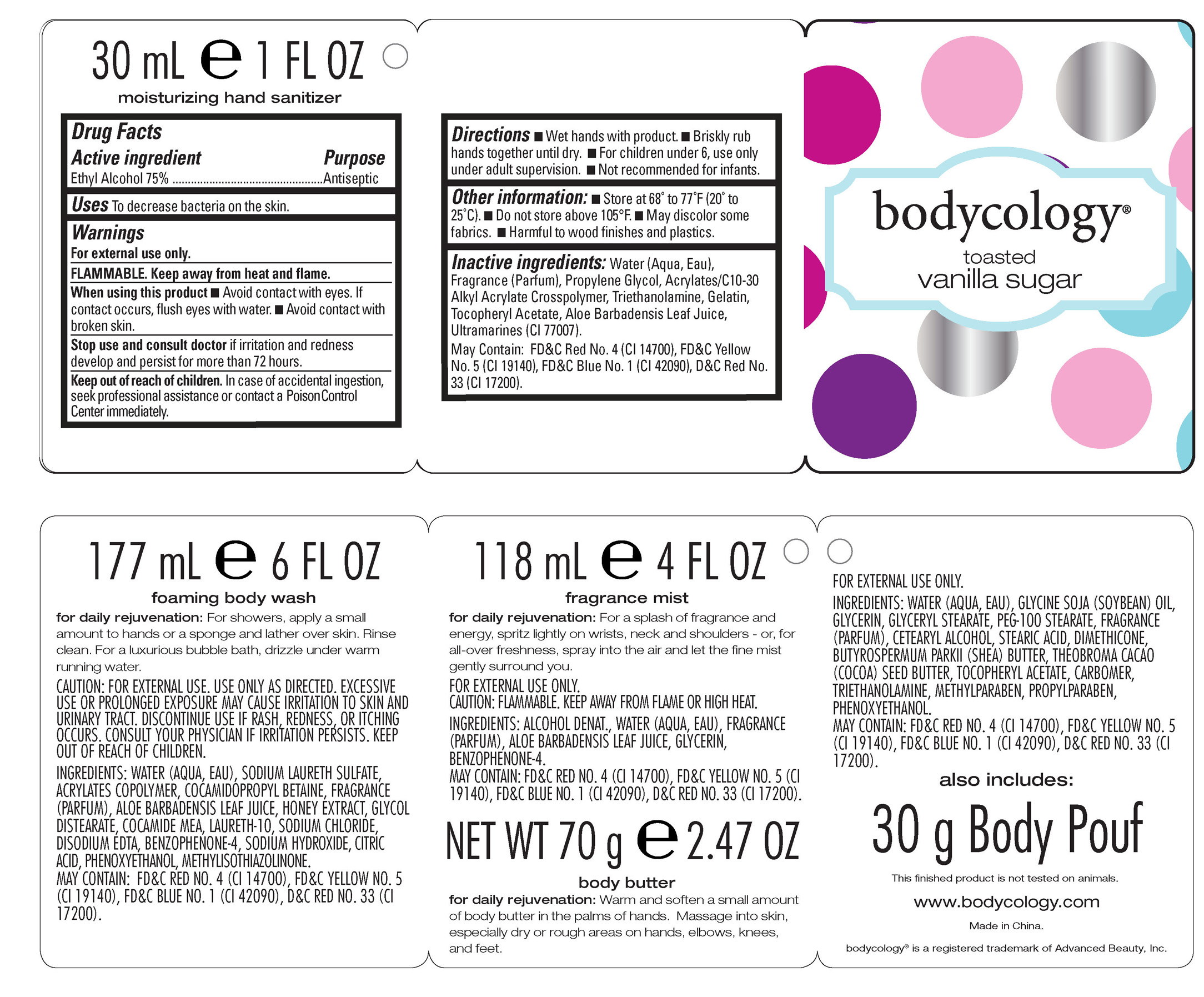
Drug Facts
Active ingredient Purpose
Ethyl Alcohol 75% Antiseptic
Uses To decrease bacteria on the skin.
Keep out of reach of children. In case of accidental ingestion, seek professional assistance or contact a Poison Control Center immediately.
Stop use and consult doctorif irritation and redness develop and persist for more than 72 hours.
Warnings
For external use only.
FLAMMABLE. Keep away from heat and flame.
When using this product
-Avoid contact with eyes. If contact occurs, flush eyes with water.
-Avoid contact with broken skin.

Directions
-Wet hands with product.
-Briskly rub hands together until dry.
-For children under 6, use only under adult supervision.
-Not recommended for infants.

Inactive ingredients: Water (Aqua, Eau), Fragrance (Parfum), Propylene Glycol, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Triethanolamine, Gelatin, Tocopheryl Acetate, Aloe Barbadensis Leaf Juice, Ultramarines (CI 77007).
May Contain: FD&C Red No. 4 (CI 14700), FD&C Yellow No. 5 (CI 19140), FD&C Blue No. 1 (CI 42090), D&C Red No. 33 (CI 17200).
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BODYCOLOGY TOASTED VANILLA SUGAR KIT
toasted vanilla sugar kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51345-065 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51345-065-01 1 in 1 KIT Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE 30 mL Part 2 1 BOTTLE 177 mL Part 3 1 BOTTLE 118 mL Part 4 1 BOTTLE 70 g Part 1 of 4 BODYCOLOGY TOASTED VANILLA SUGAR HAND SANITIZER
ethyl alcohol gelProduct Information Item Code (Source) NDC:51345-066 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 22.5 mL in 22.5 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) TROLAMINE (UNII: 9O3K93S3TK) GELATIN (UNII: 2G86QN327L) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ALOE VERA LEAF (UNII: ZY81Z83H0X) ULTRAMARINE BLUE (UNII: I39WR998BI) FD&C RED NO. 4 (UNII: X3W0AM1JLX) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) D&C RED NO. 33 (UNII: 9DBA0SBB0L) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51345-066-01 30 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 06/30/2012 Part 2 of 4 BODYCOLOGY FOAMING BODY WASH
body and hand (excluding shaving preparations)Product Information Other Ingredients Ingredient Kind Ingredient Name Quantity INGR WATER (UNII: 059QF0KO0R) INGR SODIUM LAURETH SULFATE (UNII: BPV390UAP0) INGR COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) INGR ALOE VERA LEAF (UNII: ZY81Z83H0X) INGR HONEY (UNII: Y9H1V576FH) INGR GLYCOL DISTEARATE (UNII: 13W7MDN21W) INGR COCO MONOETHANOLAMIDE (UNII: C80684146D) INGR LAURETH-10 (UNII: BD7AST04GA) INGR SODIUM CHLORIDE (UNII: 451W47IQ8X) INGR EDETATE DISODIUM (UNII: 7FLD91C86K) INGR SULISOBENZONE (UNII: 1W6L629B4K) INGR SODIUM HYDROXIDE (UNII: 55X04QC32I) INGR CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) INGR FD&C RED NO. 4 (UNII: X3W0AM1JLX) INGR FD&C YELLOW NO. 5 (UNII: I753WB2F1M) INGR FD&C BLUE NO. 1 (UNII: H3R47K3TBD) INGR D&C RED NO. 33 (UNII: 9DBA0SBB0L) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 177 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 06/30/2012 Part 3 of 4 BODYCOLOGY FRAGRANCE MIST
other fragrance preparationsProduct Information Other Ingredients Ingredient Kind Ingredient Name Quantity INGR ALCOHOL (UNII: 3K9958V90M) INGR WATER (UNII: 059QF0KO0R) INGR ALOE VERA LEAF (UNII: ZY81Z83H0X) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR SULISOBENZONE (UNII: 1W6L629B4K) INGR FD&C RED NO. 4 (UNII: X3W0AM1JLX) INGR FD&C YELLOW NO. 5 (UNII: I753WB2F1M) INGR FD&C BLUE NO. 1 (UNII: H3R47K3TBD) INGR D&C RED NO. 33 (UNII: 9DBA0SBB0L) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 118 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 07/15/2012 Part 4 of 4 BODYCOLOGY BODY BUTTER
body and hand (excluding shaving preparations)Product Information Other Ingredients Ingredient Kind Ingredient Name Quantity INGR WATER (UNII: 059QF0KO0R) INGR SOYBEAN OIL (UNII: 241ATL177A) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) INGR STEARIC ACID (UNII: 4ELV7Z65AP) INGR DIMETHICONE (UNII: 92RU3N3Y1O) INGR SHEA BUTTER (UNII: K49155WL9Y) INGR COCOA BUTTER (UNII: 512OYT1CRR) INGR .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) INGR TROLAMINE (UNII: 9O3K93S3TK) INGR METHYLPARABEN (UNII: A2I8C7HI9T) INGR PROPYLPARABEN (UNII: Z8IX2SC1OH) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR FD&C YELLOW NO. 5 (UNII: I753WB2F1M) INGR FD&C BLUE NO. 1 (UNII: H3R47K3TBD) INGR D&C RED NO. 33 (UNII: 9DBA0SBB0L) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 70 g in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 08/07/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 08/03/2012 Labeler - Advanced Beauty Systems, Inc. (129986613) Registrant - Advanced Beauty Systems, Inc. (129998613) Establishment Name Address ID/FEI Business Operations Landy International 545291775 manufacture(51345-065, 51345-066)


