BD PERSIST- povidone-iodine, alcohol solution
Becton Dickinson and Company
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
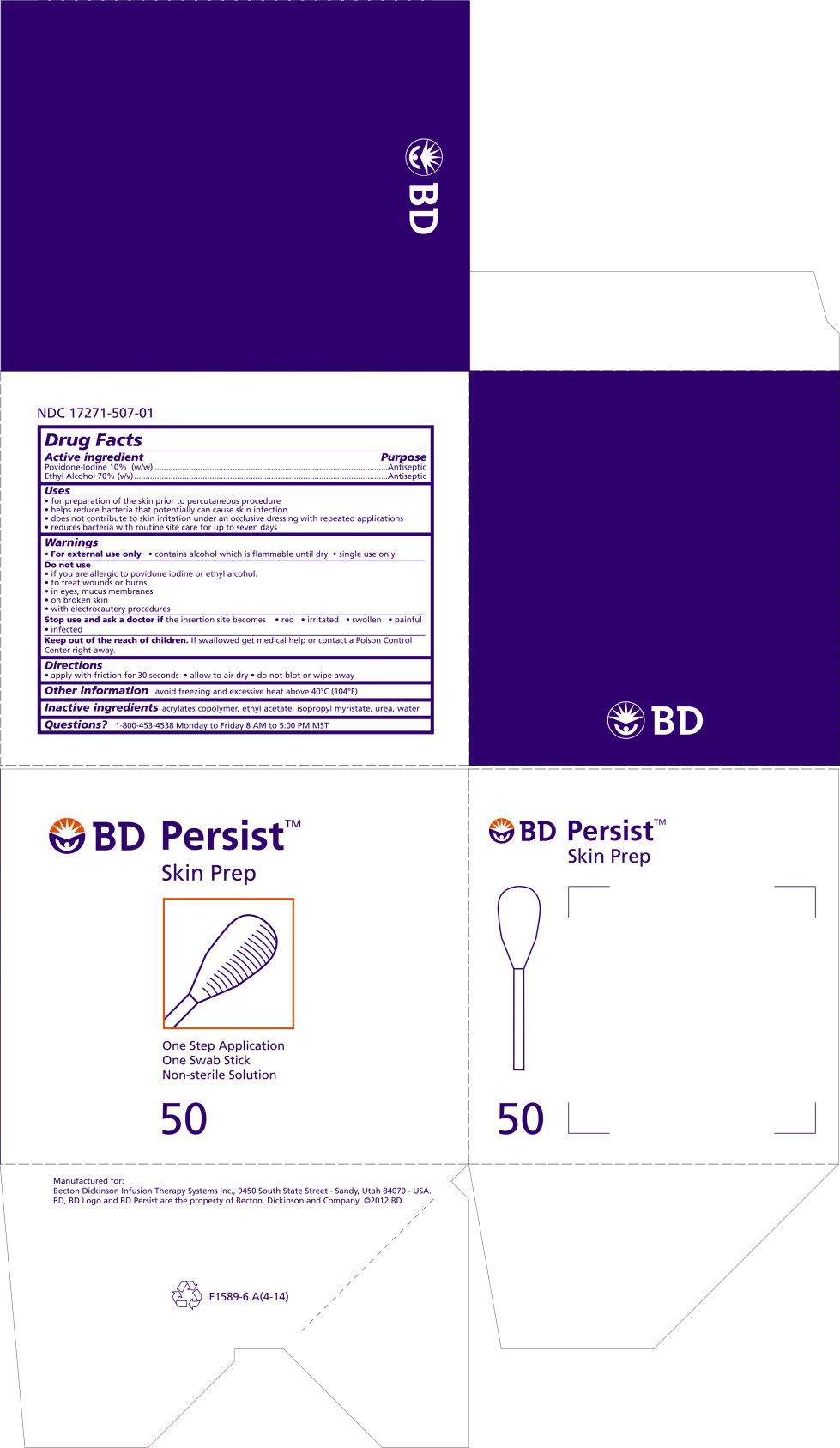
17271-507 BD Persist
Uses
- for preparation of the skin prior to percutaneous procedure
- helps reduce bacteria that potentially can cause skin infection
- does not contribute to skin irritation under an occlusive dressing with repeated applications
- reduces bacteria with routine site care for up to seven days
Warnings
- For external use only
- contains alcohol which is flammable until dry
- Single use only
| BD PERSIST
povidone-iodine, alcohol solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Becton Dickinson and Company (124987988) |
Revised: 2/2023
Document Id: f49b7ea3-1101-75e0-e053-2a95a90ab354
Set id: a2af654b-8ec6-4990-8584-742ebc597639
Version: 6
Effective Time: 20230213
Becton Dickinson and Company