Label: IBUPROFEN IMMEDIATE RELEASE- ibuprofen tablet, coated
-
Contains inactivated NDC Code(s)
NDC Code(s): 53943-401-09, 53943-401-10, 53943-401-15 - Packager: Discount Drug Mart
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 18, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT(S)
- PURPOSE
- USE(S)
-
WARNINGS
Allergy alert
Ibuprofen may cause a severe allergic reaction, especially in people allergic to aspirin. Symptoms may include:
- hives
- facial swelling
- asthma (wheezing)
- shock
- skin reddening
- rash
- blisters
If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning
This product contains an nonsteroidal anti-inflammatory drug (NSAID), which may cause severe stomach bleeding. The chance is higher if you:
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others)
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
- DO NOT USE
-
ASK A DOCTOR BEFORE USE IF
- you have problems or serious side effects from taking pain relievers or fever reducers
- the stomach bleeding warning applies to you
- you have a history of stomach problems, such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis, or kidney disease
- you have asthma
- you are taking a diuretic
- ASK A DOCTOR OR PHARMACIST BEFORE USE IF
- WHEN USING THIS PRODUCT
-
STOP USE AND ASK DOCTOR IF
- you experience any of the following signs of stomach bleeding
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present in the painful area
- any new symptoms appear
If pregnant or breast-feeding, ask a health professional before use. It is especially important not to use ibuprofen during the last 3 months of pregnancy unless definitely directed to do so by a doctor because it may cause problems in the unborn child or complications during delivery.
- you experience any of the following signs of stomach bleeding
- KEEP OUT OF REACH OF CHILDREN
-
DIRECTIONS
• do not take more than directed
• the smallest effective dose should be used
adults and children 12 years and older- take 1 tablet every 4 to 6 hours while symptoms persist
- if pain or fever does not respond to 1 tablet, 2 tablets may be used
- do not exceed 6 tablets in 24 hours, unless directed by a doctor
children under 12 years
• ask a doctor
OTHER INFORMATION- store between 20 – 25°C (68-77°F)
- tamper evident: do not use if imprinted safety seal under cap is broken or missing
- see end panel for lot number and expiry date
- INACTIVE INGREDIENT (S)
-
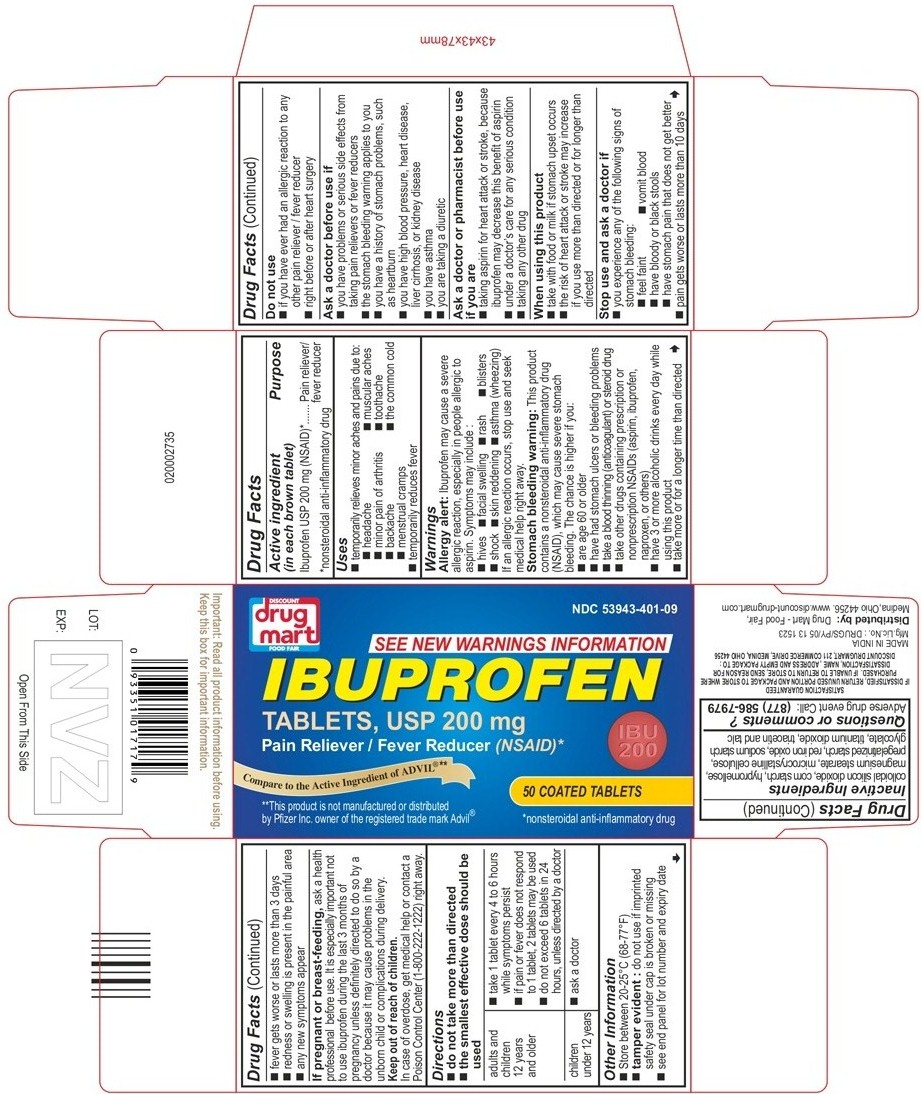
PRINCIPAL DISPLAY PANEL
Package Label (Round Shaped Tablets) - Principal Display Panel - 50s - Count Carton, 200 mg Tablets
DISCOUNT
drug mart
FOOD FAIR
NDC 53943-401-09
SEE NEW WARNINGS INFORMATION
IBUPROFEN
TABLETS, USP 200 mg
Pain Reliever / Fever Reducer (NSAID)*
IBU
200
50 COATED TABLETS
*nonsteroidal anti-inflammatory drug
Compare to the Active Ingredient of ADVIL@**
**This product is not manufactured or distributed by Pfizer Inc. owner of the registered trade mark Advil@
-
INGREDIENTS AND APPEARANCE
IBUPROFEN IMMEDIATE RELEASE
ibuprofen tablet, coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:53943-401 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IBUPROFEN (UNII: WK2XYI10QM) (IBUPROFEN - UNII:WK2XYI10QM) IBUPROFEN 200 mg Inactive Ingredients Ingredient Name Strength COLLOIDAL SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) STARCH, PREGELATINIZED CORN (UNII: O8232NY3SJ) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) TALC (UNII: 7SEV7J4R1U) FERRIC OXIDE RED (UNII: 1K09F3G675) Product Characteristics Color BROWN Score no score Shape ROUND (ROUND SHAPED) Size 10mm Flavor Imprint Code IBU200 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53943-401-09 1 in 1 CARTON 1 50 in 1 BOTTLE 2 NDC:53943-401-10 1 in 1 CARTON 2 100 in 1 BOTTLE 3 NDC:53943-401-15 500 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA079129 12/22/2014 Labeler - Discount Drug Mart (047741335) Registrant - Shasun Pharmaceuticals Limited (915786829) Establishment Name Address ID/FEI Business Operations Shasun Pharmaceuticals Limited 915786829 MANUFACTURE(53943-401)

