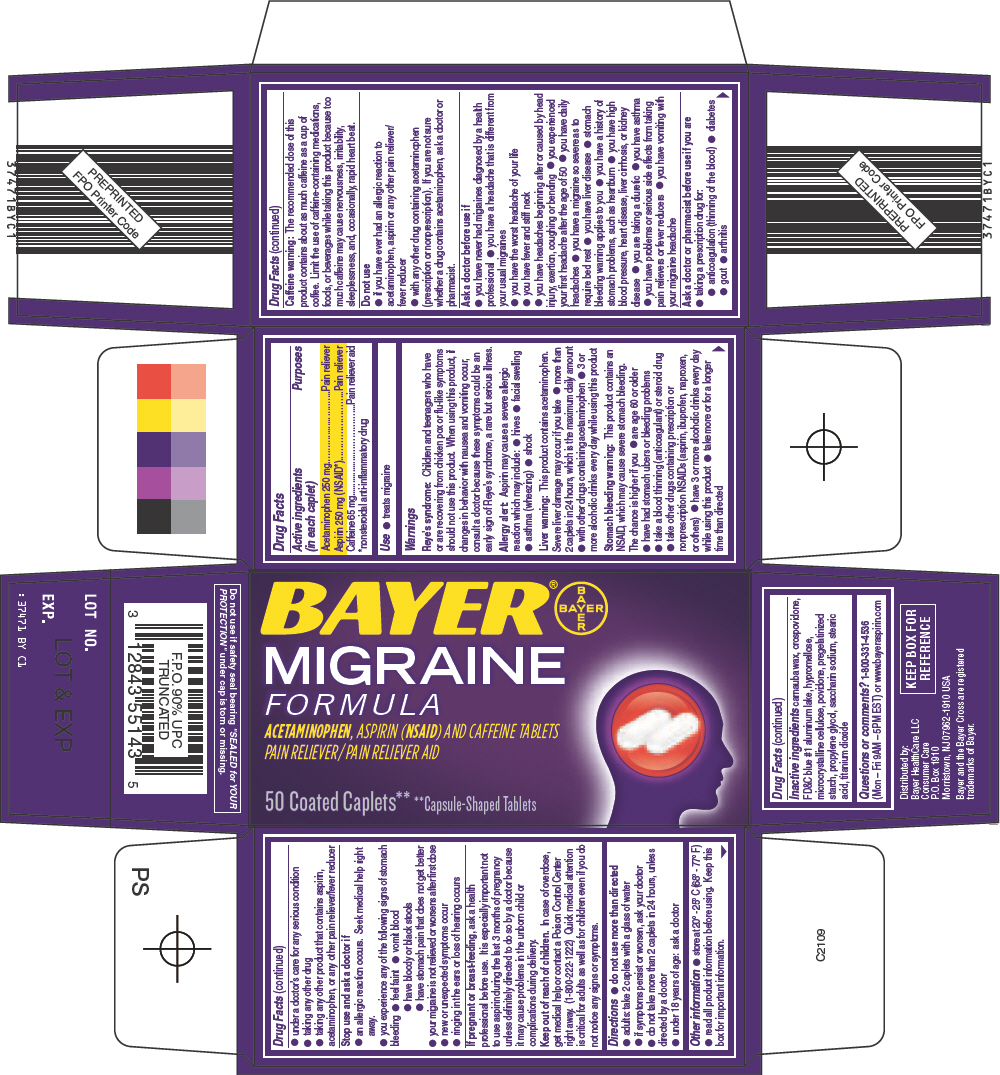
BAYER MIGRAINE FORMULA- acetaminophen, aspirin, and caffeine tablet
Bayer HealthCare LLC.
----------
Bayer Migraine Formula
Warnings
Reye's syndrome
Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert
Aspirin may cause a severe allergic reaction which may include:
- hives
- facial swelling
- asthma (wheezing)
- shock
Liver warning
This product contains acetaminophen. Severe liver damage may occur if you take
- more than 2 caplets in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Stomach bleeding warning
This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others)
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
Caffeine warning
The recommended dose of this product contains about as much caffeine as a cup of coffee. Limit the use of caffeine-containing medications, foods, or beverages while taking this product because too much caffeine may cause nervousness, irritability, sleeplessness, and, occasionally, rapid heart beat.
Do not use
- if you have ever had an allergic reaction to acetaminophen, aspirin or any other pain reliever/ fever reducer
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
Ask a doctor before use if
- you have never had migraines diagnosed by a health professional
- you have a headache that is different from your usual migraines
- you have the worst headache of your life
- you have fever and stiff neck
- you have headaches beginning after or caused by head injury, exertion, coughing or bending
- you experienced your first headache after the age of 50
- you have daily headaches
- you have a migraine so severe as to require bed rest
- you have liver disease
- stomach bleeding warning applies to you
- you have a history of stomach problems, such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis, or kidney disease
- you are taking a diuretic
- you have asthma
- you have problems or serious side effects from taking pain relievers or fever reducers
- you have vomiting with your migraine headache
Ask a doctor or pharmacist before use if you are
- taking a prescription drug for:
- anticoagulation (thinning of the blood)
- diabetes
- gout
- arthritis
- under a doctor's care for any serious condition
- taking any other drug
- taking any other product that contains aspirin, acetaminophen, or any other pain reliever/fever reducer
Stop use and ask a doctor if
- an allergic reaction occurs. Seek medical help right away.
- you experience any of the following signs of stomach bleeding
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better
- your migraine is not relieved or worsens after first dose
- new or unexpected symptoms occur
- ringing in the ears or loss of hearing occurs
Directions
- do not use more than directed
- adults: take 2 caplets with a glass of water
- if symptoms persist or worsen, ask your doctor
- do not take more than 2 caplets in 24 hours, unless directed by a doctor
- under 18 years of age: ask a doctor
Other information
- store at 20° - 25° C (68° - 77° F)
- read all product information before using. Keep this box for important information.
| BAYER MIGRAINE FORMULA
acetaminophen, aspirin, and caffeine tablet |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Bayer HealthCare LLC. (112117283) |