EQUIPTO - AMITRIPTYLINE EXTERNAL CREAM COMPOUNDING KIT- amitriptyline
Alvix Laboratories, LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Equipto-Amitriptyline External Cream Compounding Kit
For Pharmacy Prescription Compounding Only / Rx Only
For Topical Use Only
DESCRIPTION
Amitriptyline 2% External Cream Base
FOR PHARMACY PRESCRIPTION COMPOUNDING ONLY / Rx ONLY
Each Equipto - Amitriptyline External Cream Compounding Kit provides 2.4 grams of Amitriptyline powder for incorporation into 117.6 grams of a Base. The resulting mixture is intended for topical use.
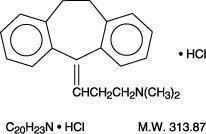
CAS-No. : 549-18-8
10,11-Dihydro-N,N-dimethyl-5 H-dibenzo[a,d] cycloheptene-Δ 5, γ-propylamine hydrochloride
WARNINGS
Pictogram


Signal word Danger Hazard statement(s)
H301 Toxic if swallowed. H361 Suspected of damaging fertility or the unborn child. Precautionary statement(s)
P201 Obtain special instructions before use.
P202 Do not handle until all safety precautions have been read and understood. P264 Wash skin thoroughly after handling.
P270 Do not eat, drink or smoke when using this product.
P281 Use personal protective equipment as required.
P301 + P310 IF SWALLOWED: Immediately call a POISON CENTER or doctor/ physician.
P308 + P313 IF exposed or concerned: Get medical advice/ attention.
P321 Specific treatment (see supplemental first aid instructions on this label).
P330 Rinse mouth. P405 Store locked up.
P501 Dispose of contents/ container to an approved waste disposal plant.
KIT COMPONENTS
Kit Components: 1 color coded teal bottle of 2.4g Amitriptyline, 1 bottle of 117.6g Base, 1 stirring rod, and 1 empty white mixing jar.
| SIZE | 120 grams |
| NDC # | 15455-0112-2 |
| Amatriptyline | 2.4 grams |
| Base | 117.6 grams |
Avoid contact with eyes. Keep container tightly closed. Keep out of reach of children. Protect from light. Dispose of the product after 30 days of being compounded.
* Certificate of analysis on file.
The FDA has not approved Equipto - Amitriptyline External Cream Compounding Kit to cure, treat, or mitigate disease.
Equipto - Amitriptyline External Cream Compounding Kit is intended for preparation in accordance with state and federal regulation governing compounding and is available to patients by prescription only.
Rx ONLY

STORAGE AND HANDLING
Prior to compounding, store Equipto - Amitriptyline External Cream Compounding Kit at room temperature between 15 - 30 degrees C (59 - 86 degrees F). Protect from light. For external use only.
| EQUIPTO - AMITRIPTYLINE EXTERNAL CREAM COMPOUNDING KIT
amitriptyline kit |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Alvix Laboratories, LLC (962445925) |




