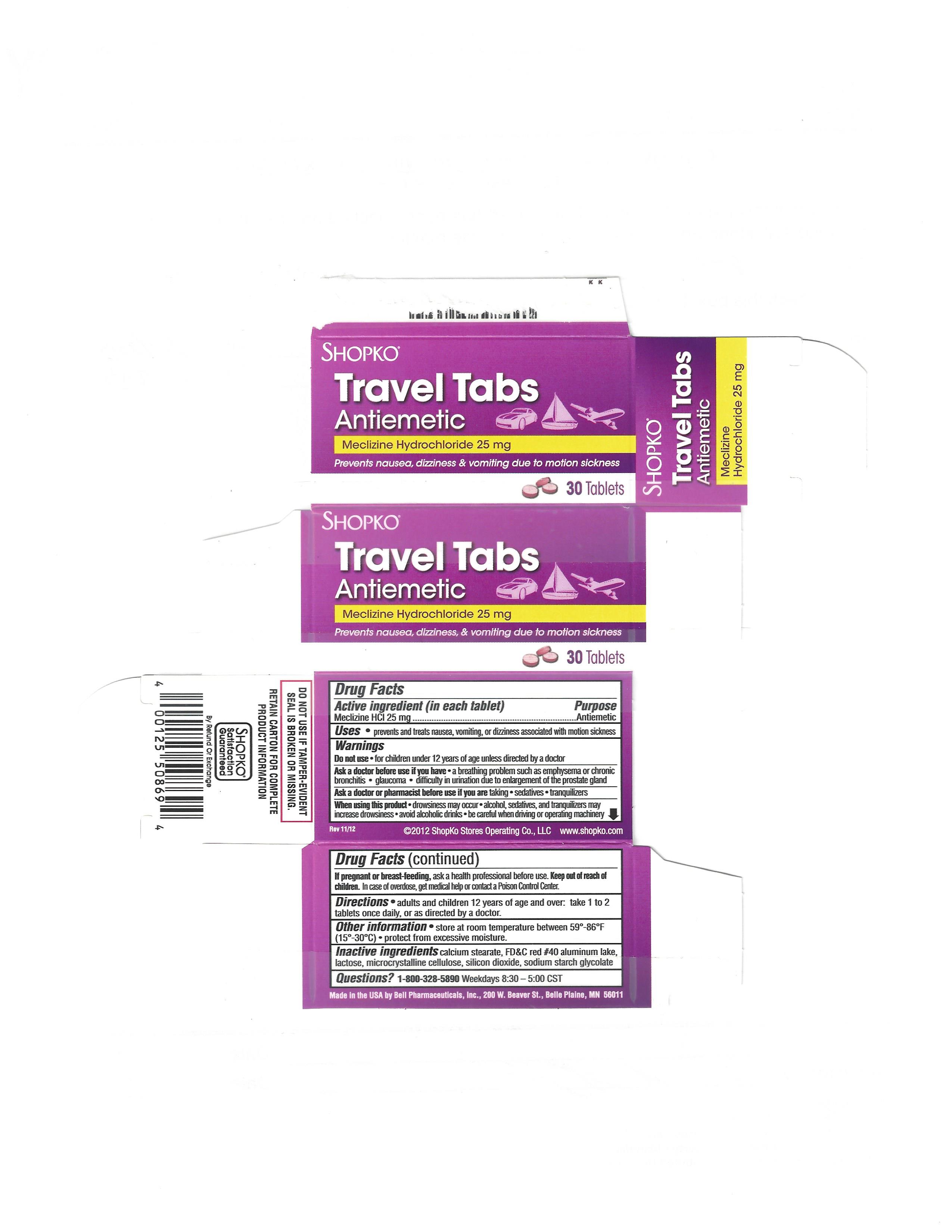
TRAV-L-TABS- meclizine hydrochloride tablet
Bell Pharmaceuticals, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
TRAV-L-TABS
Warnings
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urinating due to an enlargement of the prostate gland
Directions
- adults and children 12 years and over: take 1 to 2 tablets once daily, or as directed by a doctor.
| TRAV-L-TABS
meclizine hydrochloride tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Bell Pharmaceuticals, Inc. (140653770) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bell Pharmaceuticals, Inc. | 140653770 | manufacture(15579-826) | |
Revised: 1/2020
Document Id: 9d604249-b3fa-0427-e053-2995a90acb20
Set id: 9eeefae2-4163-4cbd-9d17-9175233718ce
Version: 5
Effective Time: 20200130
Bell Pharmaceuticals, Inc.