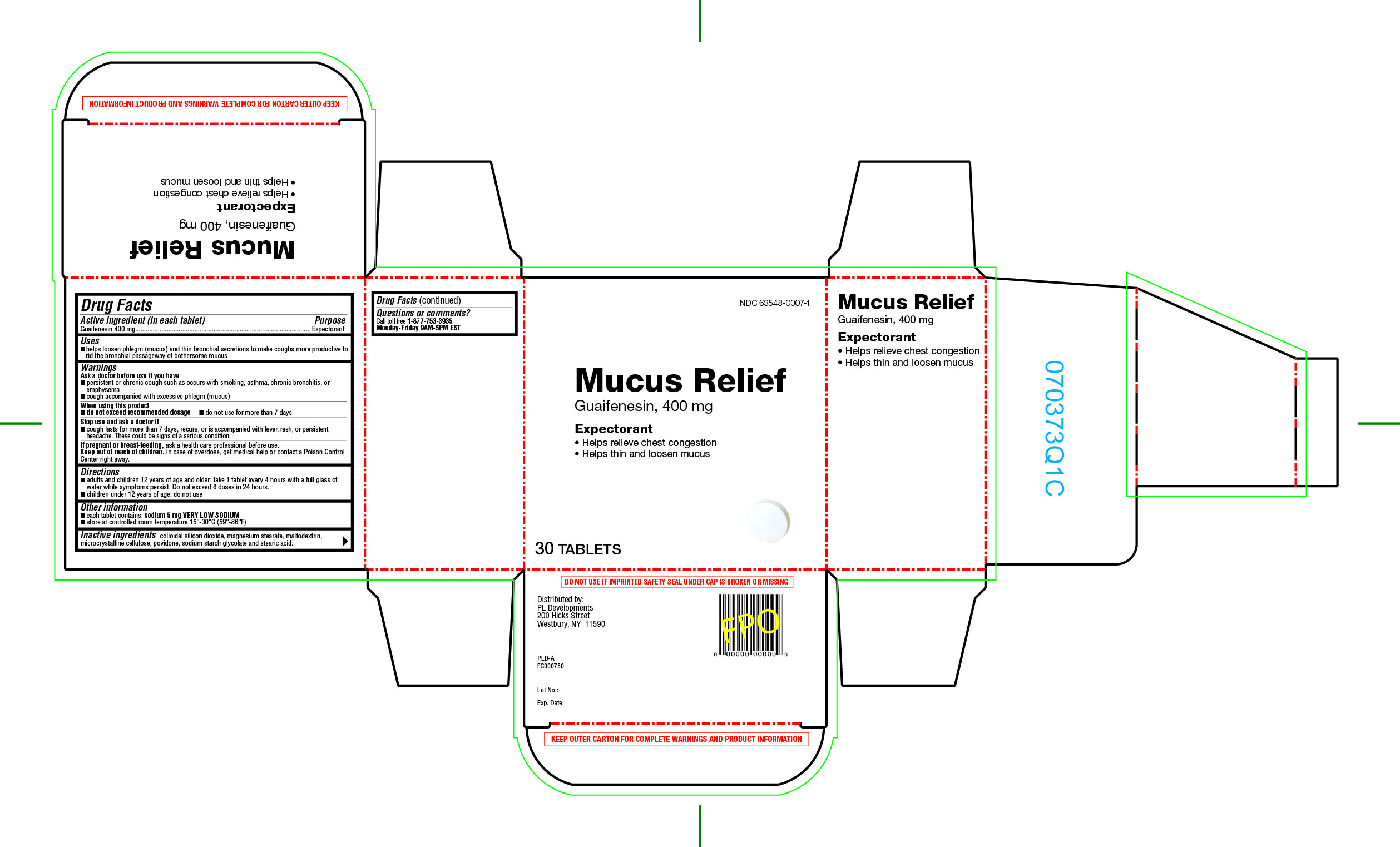
MUCUS RELIEF- guaifenesin tablet
PLD Acquisitions LLC DBA Avéma Pharma Solutions
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Avema Pharma Solutions
Uses
- helps loosen phlegm (mucus) and thin bronchial secretions to make coughs more productive to rid the bronchial passageway of bothersome mucus
Warnings
Ask a doctor before use if you have
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- cough accompanied with excessive phlegm (mucus)
Directions
- adults and children 12 years of age and older: take 1 tablet every 4 hours with a full glass of water while symptoms persist. Do not exceed more than 6 doses in 24 hours.
- children under 12 years of age: do not use
Other information
- each tablet contains: Sodium 5 mg VERY LOW SODIUM
- store at controlled room temperature 15º-30ºC (59º-86ºF)
Inactive ingredients
colloidal silicon dioxide, magnesium stearate, maltodextrin, microcrystalline cellulose, povidone, sodium starch glycolate and stearic acid.
| MUCUS RELIEF
guaifenesin tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - PLD Acquisitions LLC DBA Avéma Pharma Solutions (804087794) |
Revised: 3/2017
Document Id: 4bfc4f8a-cda9-4736-bf83-d2525f166230
Set id: 9ee3f4ee-d849-4246-9ac7-aff3145fe9fb
Version: 2
Effective Time: 20170329
PLD Acquisitions LLC DBA Avéma Pharma Solutions