URINARY INCONTINENCE RELIEF- causticum, natrum muriaticum, nus vomica, pulsatilla, sepia, staphysagria, thuja occidentalis, tablet
The Magni Company
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
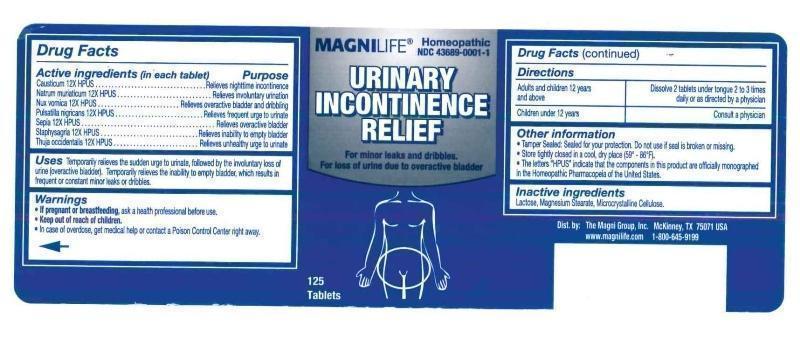
Urinary Incontinence Relief
ACTIVE INGREDIENTS: Causticum 12X, Natrum muriaticum 12X, Nux vomica 12X, Pulsatilla 12X, Sepia 12X, Staphysagria 12X, Thuja occidentalis 12X.
USES: Temporarily relieves the sudden urge to urinats, followed by the involuntary loss of urine (overactive bladder). Temporarily relieves the inability to empty bladder, which results in frequent or constant minor leaks or dribbles.
WARNINGS: If pregnant or breastfeeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
DIRECTIONS: Adults and children 12 years and over. Dissolve 2 tablets under tongue 2 to 3 times daily or as directed by a physician.
Children under 12. Consult a physician.
KEEP OUT OF REACH OF CHILDREN. In case of overdose, get medical help or contact a Poison Control Center right away.
| URINARY INCONTINENCE RELIEF
causticum, natrum muriaticum, nus vomica, pulsatilla, sepia, staphysagria, thuja occidentalis, tablet |
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
| Labeler - The Magni Company (113501902) |
| Registrant - Apotheca Company (844330915) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Apotheca Company | 844330915 | manufacture(43689-0001) | |