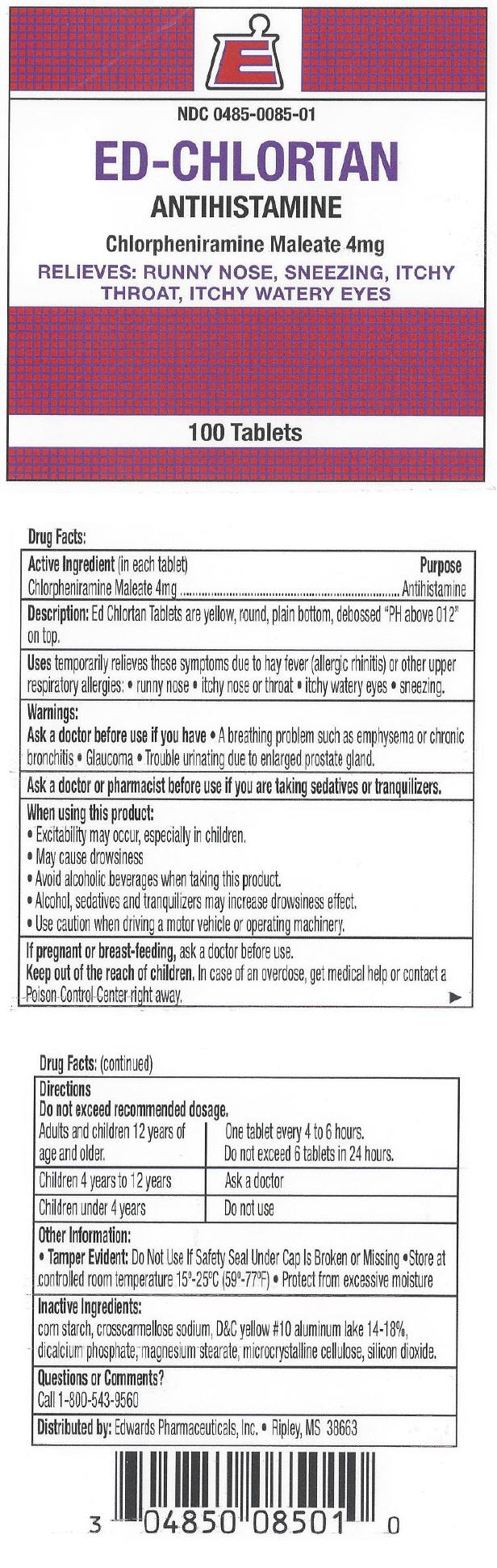
ED-CHLORTAN- chlorpheniramine maleate tablet, coated
EDWARDS PHARMACEUTICALS, INC.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
ED-CHLORTAN
Uses
temporarily relieves these symptoms due to hay fever (allergic rhinitis) or other upper respiratory allergies:
- runny nose
- itchy nose or throat
- itchy watery eyes
- sneezing.
Warnings
Ask a doctor before use if you have
- A breathing problem such as emphysema or chronic bronchitis
- Glaucoma
- Trouble urinating due to enlarged prostate gland.
Directions
Do not exceed recommended dosage.
| Adults and children 12 years of age and older. | One tablet every 4 to 6 hours. Do not exceed 6 tablets in 24 hours. |
| Children 4 years to 12 years | Ask a doctor |
| Children under 4 years | Do not use |
Other Information
- Tamper Evident: Do Not Use If Safety Seal Under Cap Is Broken or Missing
- Store at controlled room temperature 15°-25°C (59°-77°F)
- Protect from excessive moisture
| ED-CHLORTAN
chlorpheniramine maleate tablet, coated |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - EDWARDS PHARMACEUTICALS, INC. (195118880) |
Revised: 1/2022
Document Id: d57f8c18-dff0-0fd4-e053-2995a90abc70
Set id: 9ca71d93-2d32-4a74-8a5a-39ef446067e0
Version: 3
Effective Time: 20220113
EDWARDS PHARMACEUTICALS, INC.