ECOLAB- triclosan solution
Ecolab Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
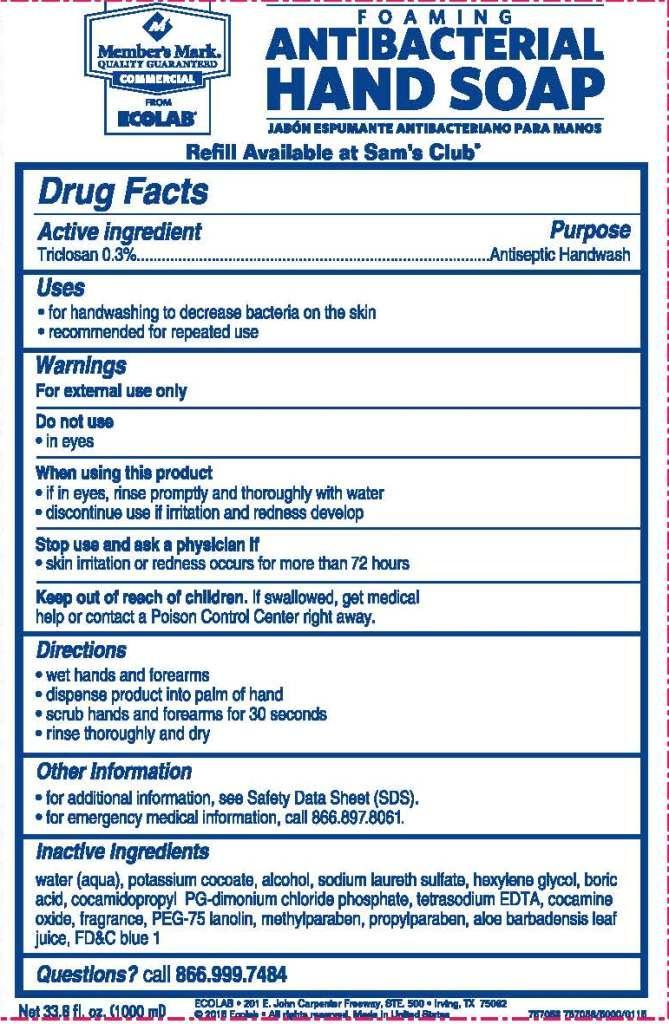
Drug Facts
Warnings
For external use only
Directions
- wet hands and forearms
- dispense product into palm of hand
- scrub hands and forearms for 30 seconds
- rinse thoroughly and dry
Other Information
- for additional information, see Safety Data Sheet (SDS).
- for emergency medical information, call 866.897.8061.
| ECOLAB
triclosan solution |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Ecolab Inc. (006154611) |
Revised: 2/2018
Document Id: 8c017267-17f1-4f4d-b744-a102192deabc
Set id: 9c602900-b14f-475d-8b45-64a6befcf3d4
Version: 2
Effective Time: 20180216
Ecolab Inc.