MOTION SICKNESS II- meclizine hcl tablet
TOP CARE (Topco Associates LLC)
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Uses
for prevention and treatment of nausea, vomiting, or dizziness associated with motion sickness.
Warnings
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
Directions
- to prevent motion sickness, the first dose should be taken 1 hour before starting activity
- adults and children 12 years and over: 1 to 2 tablets once daily, or as directed by a doctor
- do not exceed 2 tablets in 24 hours
Inactive ingredients
anhydrous lactose, colloidal silicon dioxide, corn starch, D&C yellow #10 aluminium lake, magnesium stearate, microcrystalline cellulose, sodium starch glycolate
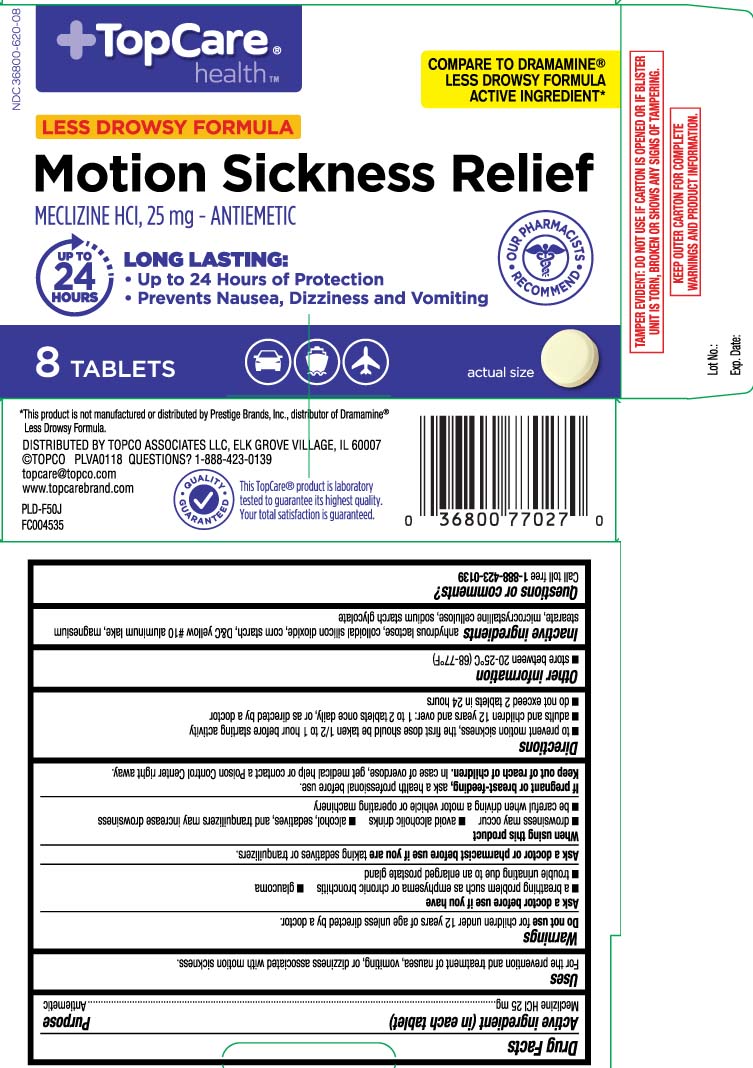
Principal Display Panel
COMPARE TO DRAMAMINE® LESS DROWSY FORMULA ACRIVE INGREDIENT*
LESS DROWSY FORMULA
Motion Sickness Relief
MECLIZINE HCI, 25 mg - ANTIEMETIC
LONG LASTING
- Up to 24 Hours of Protection
- Prevents Nausea, Dizziness and Vomiting
TABLETS
*This product is not manufactured or distributed by Prestige Brands, Inc., distributor of Dramamine® Less Drowsy Formula.
DISTRIBUTED BY TOPCO ASSOCIATES LLC
ELK GROVE VILLAGE, IL 60007
TAMPER EVIDENT: DO NOT USE IF CARTON IS OPENED OR IF BLISTER UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING.
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
| MOTION SICKNESS II
meclizine hcl tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - TOP CARE (Topco Associates LLC) (006935977) |