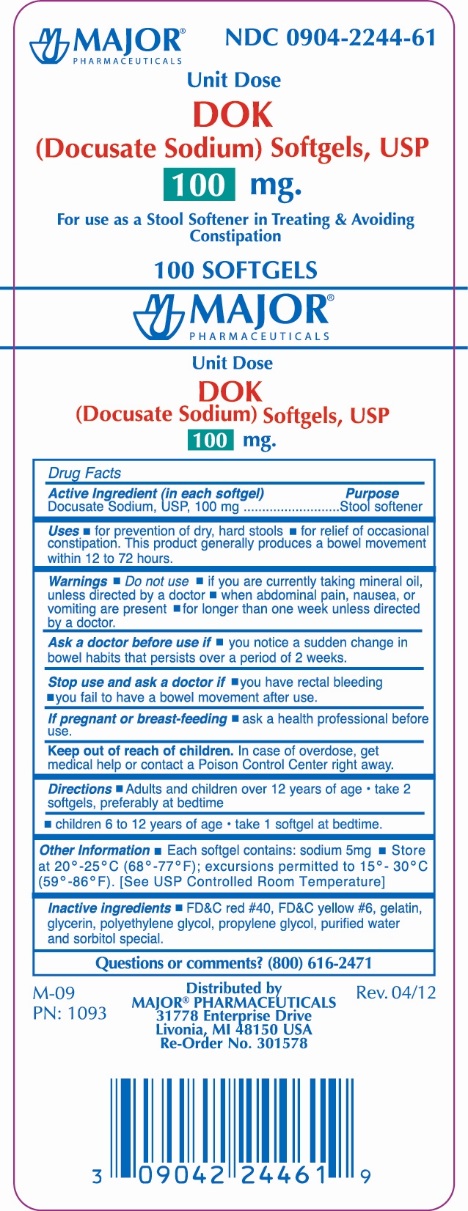
DOK- docusate sodium tablet, film coated
Major Pharmaceuticals
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Uses
- •
- for prevention of dry, hard stools
- •
- for relief of occasional constipation.This product generally produces a bowel movement within 12 to 72 hours.
Warnings
Do not use
- •
- if you are currently taking mineral oil, unless directed by a doctor
- •
- when abdominal pain, nausea, or vomiting are present
- •
- for longer than one week unless directed by a doctor
Ask a doctor before use if
- •
- you notice a sudden change in bowel habits that persists over a period of 2 weeks
Directions
|
Adults and children 12 years of age: |
Take 2 softgels, preferably at bedtime |
|
Children 6 to 12 years of age |
Take 1 softgel at bedtime |
Other information
- •
- Each softgel contains: sodium 5mg
- •
- Store at 20º - 25ºC (68º - 77ºF); excursions permitted to 15º - 30ºC (59º - 86ºF). [See USP Controlled Room Temperature]
| DOK
docusate sodium tablet, film coated |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Major Pharmaceuticals (191427277) |
Revised: 12/2019
Document Id: 07ed0d89-9d76-4ed5-8d0e-6f002243966e
Set id: 9ac7514b-fa72-46be-b932-ca69fe7ae07b
Version: 3
Effective Time: 20191213
Major Pharmaceuticals