SHEERFLUORX FLUORIDE TREATMENT- sodium fluoride film
CAO Group, Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Product Information
Sheer FluorX™ fluoride treatment is the ideal way to make in-office fluoride treatments easy, faster, more comfortable, and more practical. Kids will love Sheer FluorX – no more trays, foams or gels that cause gagging and mess. The sheer film molds to teeth delivering fluoride exactly where you want it, eliminating foam running down the throat and being swallowed. The 5% sodium fluoride acidulated formula provides an effective and long duration application of fluoride to the teeth.
- Do not smoke during the fluoride treatment.
- Avoid eating or drinking while wearing the film.
- Flush with copious amounts of water if accidentally
placed in eye. - Keep out of reach of young children.
- Not for use by children under the age of 12.
- Avoid contact with clothing.
- Do not use if you have a known allergy to fluoride.
- Do not wear for more than 15 minutes in a 24 hour period.
- Attention - Refer to the instructions for use and complete information.
- Use only as directed. Keep out of reach of small children and animals.
- Storage Temperature - Store product at the indicated temperature (32°F/0°C to 100°F/38°C).
- Do not store above 100°F (38°C). Do not freeze. Do not use beyond indicated shelf life.
- Do not use if the packaging has been damaged, or if the seals are found to be broken or open.
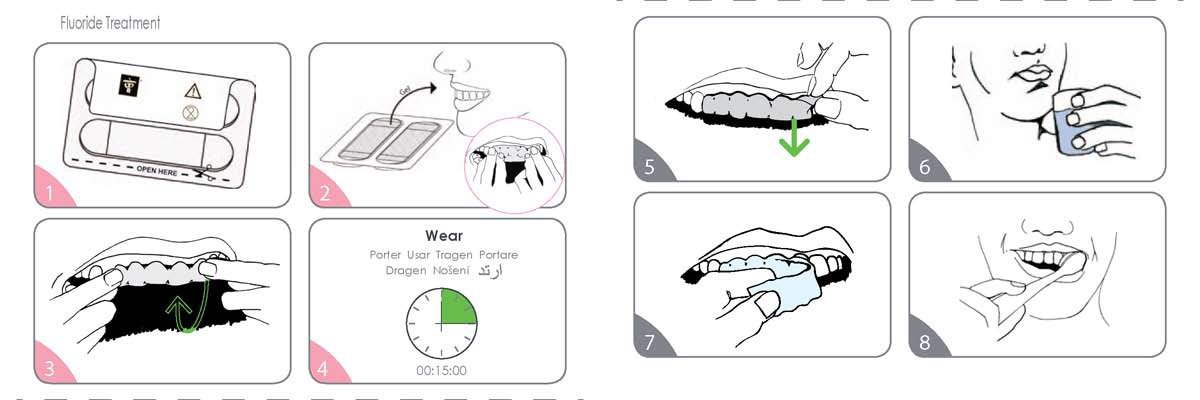
- Separate the paper layer from the plastic tray by pulling where indicated. The sticky gel side is against the plastic layer.
- Remove shrink wrap from tray. Carefully remove film from tray. The sticky gel side faces up. Position the film so it is centered on the arch. Firmly apply the film to the teeth, with the edge of the film just extending onto the gums.
- Starting at the front teeth, wrap the film around the edge of the teeth and onto the back side of the teeth. Use your fingers to firmly mold the film to your teeth.
- Leave the film in place for 15 minutes. DO NOT wear for more than 15 minutes in a 24 hour period.
- Grasp corner of film and begin to loosen away from teeth.
- If film becomes difficult to remove, rinse with water.
- Continue removing film from all surfaces. The film may come away in pieces; this is normal.
- Brush teeth and gums to remove any remaining gel.
COMPLETE INSTRUCTIONS - Back Page
Caution: Federal law restricts this device to sale by or on the order of a licensed professional.
Disclaimer: CAO Group believes this information to be accurate and is offered only for the benefit of its customers for use of the product under proscribed conditions. This document is not to be considered a warranty or guarantee of product performance, and CAO Group is not legally bound to such claims based on this document.
Manufactured by CAO (China) Medical Equipment Co,. Ltd. for CAO Group. Inc.
P 877.236.4408 F 801.256.9287
4628 West Skyhawk Drive, West Jordan, UT 84084-4501 U.S.A.
www.caogroup.com
SAL-CAI011B.1/28SEP2010
FRONT PANEL
SheerFluorx
Fluoride Treatment
Chairside Strength - Home Applied.
Disclaimer: CAO Group believes this information to be accurate and is offered only for the benefit of its customers for use of the product under proscribed conditions. This document is not to be considered a warranty or guarantee of product performance, and CAO Group is not legally bound to such claims based on this document.
Shelf Life: 2 years
Manufactured by CAO (China) Medical Equipment Co,. Ltd. for CAO Group, Inc.
Phone 877.236.4408 Fax 801.256.9287
4628 West Skyhawk Drive, West Jordan, UT 84084-4501 U.S.A.
www.caogroup.com
SAL-PTK002A.1/28SEP2010
IMMEDIATE PACKAGE DISPLAY PANEL
SheerFluorX
Fluoride Treatment
Chairside Strength - Home Applied.
LOT: [lot number]
EXP: [expiration date]
CAO Group, Inc.
Easier. Faster. Better.
TO REORDER:
Henry Schein
1.800.372.4346
Storage
[32°F/0°C to 100°F/38°C]
See carton for complete instructions and information. Keep out of reach of children.
For one-time use only.
PAK-LA0016C.1/28SEP2010
OUTER PACKAGE - Back panel
TO REORDER: Henry Schein 1.800.372.4346
PACKAGE CONTENTS: 4) fluoride treatment packs, each containing 2 applicator films
See package insert for dosage information. Rx Only.
Dispose of properly after use. For complete safety information see product MSDS.
Do not use if the packaging has been damaged, or if the safety seals are found to be broken.
REORDER # 006-00103
Manufactured by CAO (China) Medical Equipment Co., Ltd. for CAO Group, Inc.
4628 West Skyhawk Drive
West Jordan, UT 84084
U.S.A.
877-236-4408 (tel)
801-256-9287 (fax)
www.caogroup.com
STORAGE: [32°F/0°C to 100°F/38°C]
Expiration Date: [lot and expiration label]
[Barcode: 872320000820]
MADE IN CHINA
| SHEERFLUORX
FLUORIDE TREATMENT
sodium fluoride film |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - CAO Group, Inc. (102422578) |