SINUS AND ALLERGY RELEIF PE- chlorpheniramine maleate, phenylephrine hcl tablet
P & L Development, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
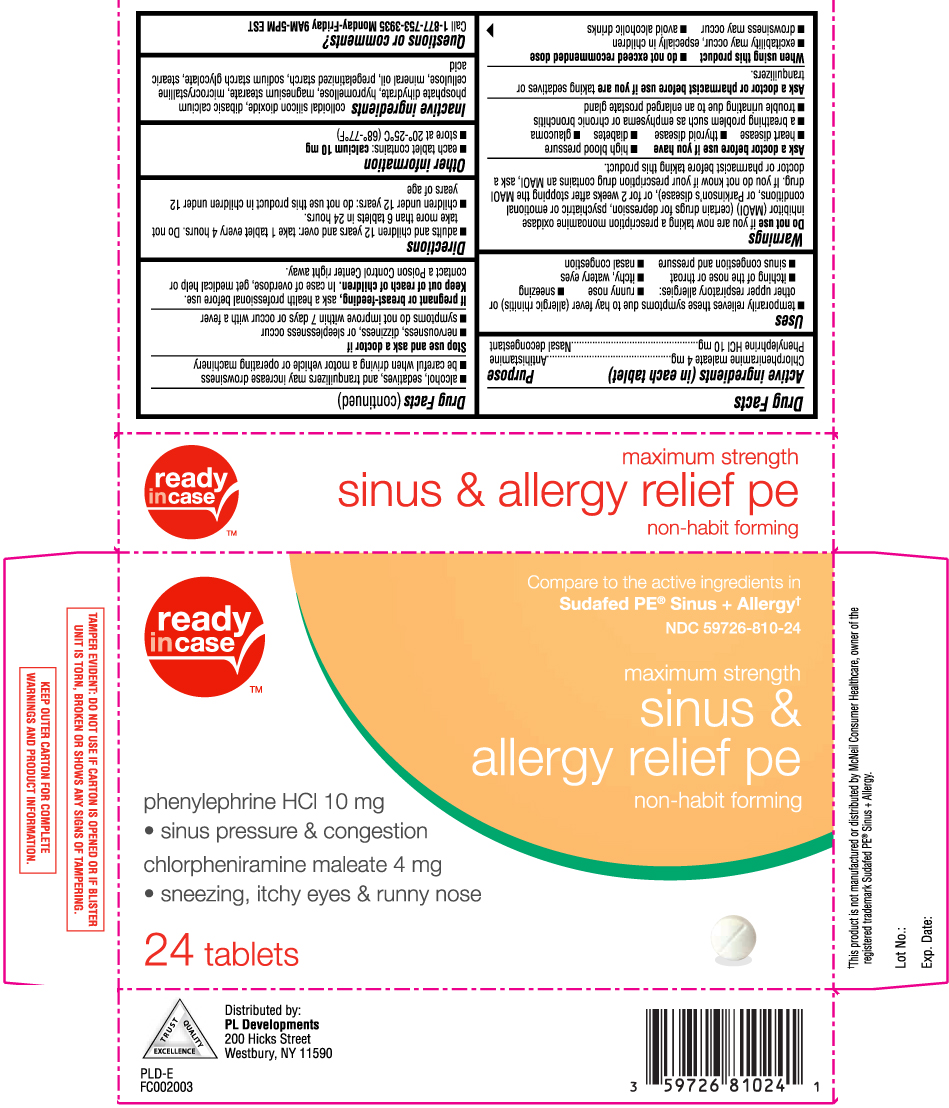
Drug Facts
Uses
- temporarily relieves these symptoms due to hay fever (allergic rhinitis) or other upper respiratory allergies:
- runny nose
- sneezing
- itching of the nose or throat
- itchy, watery eyes
- sinus congestion and pressure
- nasal congestion
Warnings
Do not use
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- high blood pressure
- heart disease
- thyroid disease
- diabetes
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
- trouble urinating due to an enlarged prostate gland
When using this product
- do not exceed recommended dose
- excitability may occur, especially in children
- drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
Directions
- adults and children 12 years and over: take 1 tablet every 4 hours. Do not take more than 6 tablets in 24 hours.
- children under 12 years: do not use this product in children under 12 years of age
Inactive ingredients
colloidal silicon dioxide, dibasic calcium phosphate dihydrate, hypromellose, magnesium stearate, microcrystalline cellulose, mineral oil, pregelatinized starch, sodium starch glycolate, stearic acid
Principal Display Panel
Compare to the active ingredients in Sudafed PE® Sinus + Allergy†
Maximum Strength
Sinus & Allergy Relief PE
Non-Habit Forming
Phenylephrine HCl 10 mg
- Sinus Pressure & Congestion
Chlorpheniramine maleate 4 mg
- Sneezing, Itchy Eyes & Runny Nose
Tablets
†This product is not manufactured or distributed by McNeil Consumer Healthcare, owner of the registered trademark Sudafed PE® Sinus + Allergy.
Distributed by: PL Developments
200 Hicks Street, Westbury, NY 11590
TAMPER EVIDENT: DO NOT USE IF CARTON IS OPENED OR IF BLISTER UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING.
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
| SINUS AND ALLERGY RELEIF PE
chlorpheniramine maleate, phenylephrine hcl tablet |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - P & L Development, LLC (800014821) |