MCKESSON FOAMING HAND CLEANSE- triclosan soap
McKesson Medical-Surgical Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
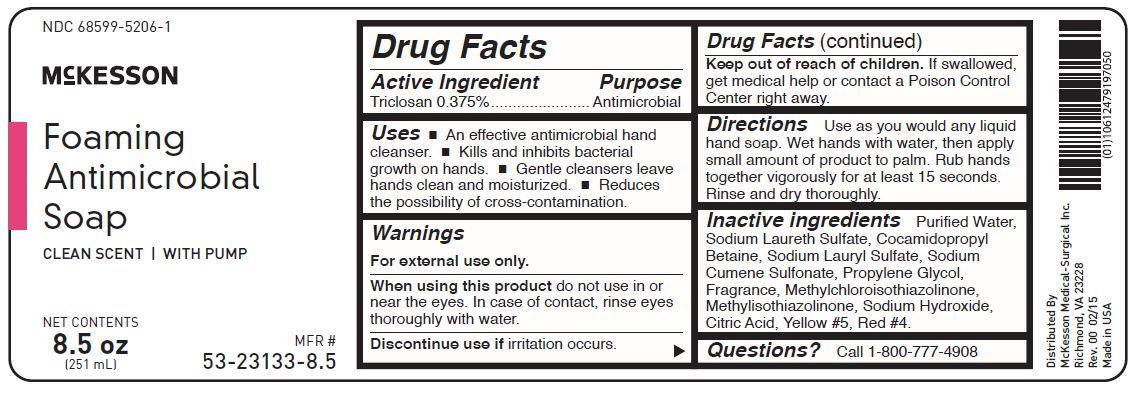
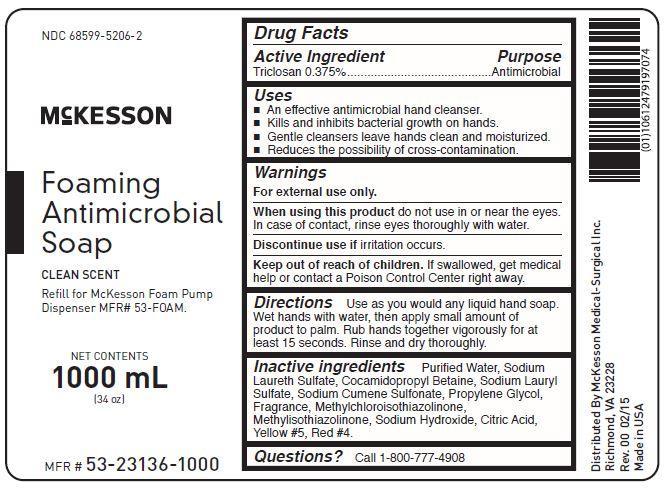
Uses
- an effective antimicrobial hand cleanser
- kills and inhibits bacterial growth on hands
- gentle cleansers leave hands clean and moisturized
- reduces the possibility of cross-contamination
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
McKesson Foaming Antimicrobial Soap
Clean Scent | With Pump
Net Contents 8.5 oz (251 mL)
Questions? Call 1-800-777-4908
Directions
Use as you would any liquid hand soap. Wet hands with water, then apply small amount of product to palm. Rub hands together vigorously for at least 15 seconds. Rinse and dry thoroughly.
Warnings
For external use only.
When using this product do not use in or near the eyes. In case of contact, rinse eyes thoroughly with water.
Discontinue use if irritation occurs.
| MCKESSON FOAMING HAND CLEANSE
triclosan soap |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - McKesson Medical-Surgical Inc. (023904428) |