Label: EUCERIN ECZEMA RELIEF BODY- oatmeal cream
-
NDC Code(s):
10356-365-02,
10356-365-23,
10356-365-34,
10356-365-35, view more10356-365-37, 10356-365-56
- Packager: Beiersdorf Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 29, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- WARNINGS
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- DOSAGE & ADMINISTRATION
- QUESTIONS
-
INACTIVE INGREDIENT
Inactive Ingredients
Water, Glycerin, Ricinus Communis (Castor) Seed Oil,
Mineral Oil, Cetyl Alcohol, Glyceryl Stearate, Caprylic/Capric Triglyceride,
Octyldodecanol, Cetyl Palmitate, PEG-40 Stearate, Glycyrrhiza Inflata
Root Extract, Ceramide 3, 1,2-Hexanediol, Phenoxyethanol, Piroctone
Olamine, Caprylyl Glycol, Ethylhexylglycerin, Benzyl Alcohol, Citric Acid. - PURPOSE
-
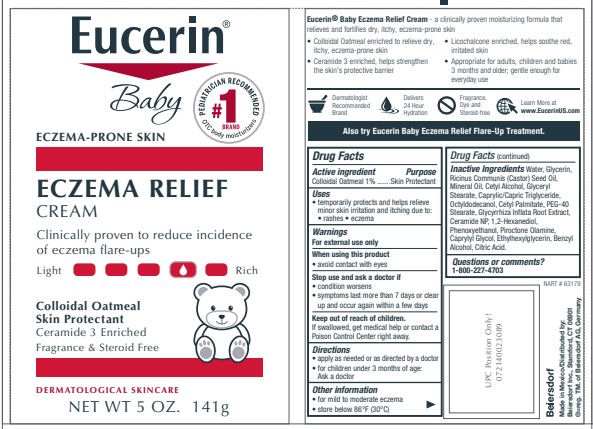
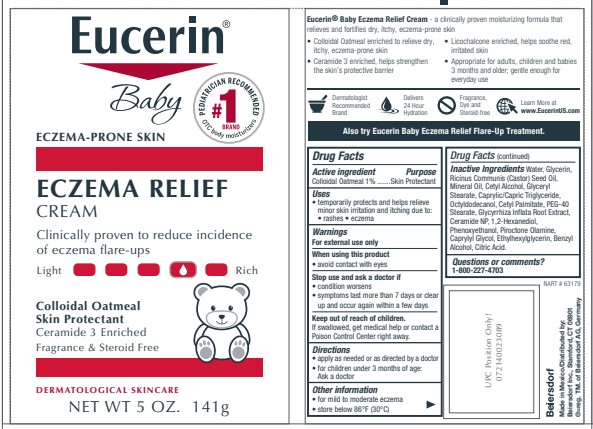
PRINCIPAL DISPLAY PANEL
Eucerin
Eczema Relief Body Creme
Colloidal Oatmeal Skin Protectant
Ceramide 3 enriched
Clinically proven to reduce incidence of eczema flare ups
Steroid Free and Fragrance Free

Eucerin Baby
Eczema Relief Body Creme
Oatmeal and Ceramide-3 enriched
daily moisturizer clinically proven to fortify dry, itchy,
eczema prone skin
Skin Protectant
Steroid Free and Fragrance Free
-
INGREDIENTS AND APPEARANCE
EUCERIN ECZEMA RELIEF BODY
oatmeal creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10356-365 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OATMEAL (UNII: 8PI54V663Y) (OATMEAL - UNII:8PI54V663Y) OATMEAL 1 g in 100 g Inactive Ingredients Ingredient Name Strength CAPRYLYL GLYCOL (UNII: 00YIU5438U) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) BENZYL ALCOHOL (UNII: LKG8494WBH) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) WATER (UNII: 059QF0KO0R) PIROCTONE OLAMINE (UNII: A4V5C6R9FB) GLYCERIN (UNII: PDC6A3C0OX) CASTOR OIL (UNII: D5340Y2I9G) MINERAL OIL (UNII: T5L8T28FGP) CETYL ALCOHOL (UNII: 936JST6JCN) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) OCTYLDODECANOL (UNII: 461N1O614Y) CETYL PALMITATE (UNII: 5ZA2S6B08X) PEG-40 STEARATE (UNII: ECU18C66Q7) GLYCYRRHIZA INFLATA ROOT (UNII: 1MV1Z7MKVQ) CERAMIDE 3 (UNII: 4370DF050B) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) PHENOXYETHANOL (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10356-365-34 226 g in 1 TUBE; Type 0: Not a Combination Product 07/15/2013 2 NDC:10356-365-35 141 g in 1 TUBE; Type 0: Not a Combination Product 07/15/2013 3 NDC:10356-365-23 10 g in 1 TUBE; Type 0: Not a Combination Product 07/15/2013 4 NDC:10356-365-02 7 g in 1 PACKET; Type 0: Not a Combination Product 07/15/2013 5 NDC:10356-365-37 396 g in 1 TUBE; Type 0: Not a Combination Product 04/15/2020 6 NDC:10356-365-56 3 in 1 PACKAGE 08/15/2021 6 NDC:10356-365-34 226 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 07/15/2013 Labeler - Beiersdorf Inc (001177906)